 iRhythm Technologies has announced two US Food and Drug Administration (FDA) 510K clearances—one for a new and improved design of its flagship monitor and a second for updated artificial intelligence (AI) capabilities.
iRhythm Technologies has announced two US Food and Drug Administration (FDA) 510K clearances—one for a new and improved design of its flagship monitor and a second for updated artificial intelligence (AI) capabilities.
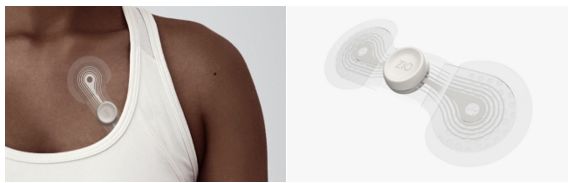
The new Zio monitor is designed to significantly improve patient comfort while the advancements to its AI capabilities are intended to improve rhythm and beat diagnostic accuracy.
“iRhythm’s new Zio monitor and enhanced AI further illustrates our commitment to raising the standard of cardiac care for the providers and patients we serve,” said Mike Coyle, CEO of iRhythm. “By improving patient comfort and experience we can continue to maximise patient compliance, which is an essential element for collecting high-quality data for analysis. And with the power of our next generation AI, we can help physicians better identify, diagnose and manage a wide array of significant arrhythmias, including atrial fibrillation.”
The new Zio monitor is designed to be easy to wear with increased adherence and better patient comfort. The new design is more than 50% lighter than the current generation and includes a new breathable and waterproof outer layer. It also has a ‘stay-put’ adhesive and a more flexible design for a secure attachment.
“iRhythm started with the patient in mind first,” said Judy Lenane, chief clinical officer and executive vice president of products at iRhythm. “We asked ourselves what it would take to give patients the best, most comfortable experience, and designed it from there. We’re excited to offer more patients around the world access, as well as an improved patient experience, which will enable far more insight into the data.”
iRhythm has collected over 750 million hours of curated heartbeat data, creating the largest repository of labelled ECG patient data in the world. This allows for expanded training of the algorithm across a larger database, the company says in a press release, resulting in enhanced AI diagnostic accuracy and better quality assurance.
“With this clearance, we are excited to be further improving on our AI-based detection capabilities, raising the bar for the cardiac monitoring industry,” said Mark Day, executive vice president of research and development at iRhythm. “This new AI algorithm will enable the Zio Service to be even more accurate and scalable, delivering more clinical insight for cardiologists and payers.”
The updated AI capabilities will be introduced later this quarter for the US-based Zio service, while the new Zio monitor platform will begin limited release later this year.












