Tag: Medtronic
Medtronic’s Sphere-360 ablation catheter gains CE mark alongside first uses in...
Medtronic has today announced two major milestones relating to the Affera Sphere-360 catheter—the receipt of a CE mark in Europe and completion of the first cases...
Medtronic announces US commercial launch of OmniaSecure defibrillation lead
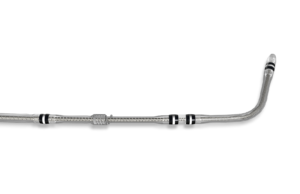
Medtronic has commercially launched the OmniaSecure defibrillation lead in the USA this week, with the first cases being performed at hospitals across the country.
OmniaSecure...
Medtronic shares latest Enlighten and LEADR study results at APHRS 2025
Medtronic has announced the recent presentation of results from two late-breaking studies at the 2025 Asia Pacific Heart Rhythm Society (APHRS) Scientific Sessions (12–15...
Medtronic initiates global study examining cardiac pacing in HFpEF patients
Medtronic has announced the initiation of a pivotal study evaluating the use of elevated and personalised cardiac pacing rates for the treatment of patients...
Multiple datasets suggest positive safety and performance with OmniaSecure defibrillation lead
Researchers recently presented three new datasets reinforcing the safety and performance of Medtronic’s OmniaSecure defibrillation lead at the 2025 European Society of Cardiology (ESC)...
Abbott, Medtronic announce positive data on respective PFA systems at HRS...
Among a great number of presentations relating to pulsed field ablation (PFA) treatments for atrial fibrillation (AF) at this year’s Heart Rhythm Society (HRS)...
Medtronic receives US FDA approval for OmniaSecure lead and announces investigational...
Medtronic has received US Food and Drug Administration (FDA) approval of the OmniaSecure defibrillation lead for placement within the right ventricle. The lead—based on...
Medtronic announces first patient enrolment in trial assessing VT treatment with...
Medtronic recently announced that the first patient has been enrolled in a US Food and Drug Administration (FDA) early feasibility study evaluating the Affera...
DiVERT Stroke study uncovers sex-based disparities in post-stroke cardiac care
New findings from the DiVERT Stroke clinical study show that women spent less time in the hospital, saw fewer referrals to cardiology and were...
Cardiac Rhythm News’ top stories of 2024
From first-time clinical data to major industry debuts, here are Cardiac Rhythm News’ 10 most popular stories of 2024.
1. Abbott joins the pulsed field...
LINQ family of AI-supported insertable cardiac monitors accurately predicts risk thresholds...
Primary results from the DEFINE AFib clinical study have found that Medtronic's LINQ family of insertable cardiac monitors (ICMs), paired with a novel algorithm,...
Texas Cardiac Arrhythmia Institute becomes first in USA to use FDA-approved...
Electrophysiologists at the Texas Cardiac Arrhythmia Institute (TCAI) at St David's Medical Center (Austin, USA) recently became the first in the USA to use...
Medtronic receives US FDA approval of Affera mapping and ablation system...
Medtronic has announced US Food and Drug Administration (FDA) approval of the Affera mapping and ablation system with Sphere-9 catheter, an all-in-one, high-density (HD)...
New clinical data show “excellent lesion durability” with Medtronic’s PulseSelect PFA...
Medtronic has announced the presentation of clinical study results demonstrating a high rate of durable lesion formation with the PulseSelect pulsed field ablation (PFA)...
Final results from Medtronic’s EV ICD pivotal trial demonstrate high ATP...
Medtronic recently shared long-term results from the global EV ICD pivotal trial, reinforcing the performance and safety of the company’s extravascular implantable cardioverter defibrillator...
Viz.ai and Medtronic to collaborate on improving post-cryptogenic stroke care in...
Viz.ai has announced a strategic collaboration with Medtronic to improve the coordination of cryptogenic stroke patient care between neurology and cardiology teams.
For stroke patients...
US FDA grants Medtronic approval for extravascular defibrillator to treat abnormal...
Medtronic has received US Food and Drug Administration (FDA) approval for the Aurora EV-ICD MRI SureScan (extravascular implantable cardioverter-defibrillator) and Epsila EV MRI SureScan defibrillation...
Amazon executive joins Medtronic to spearhead development in robotics and implantables
Medtronic has announced that Ken Washington has been appointed chief technology and innovation officer.
Washington joins Medtronic from Amazon where he served as vice president...
Medtronic mapping and ablation system shows “safety and performance” in treating...
Medtronic have announced the 12-month findings that supported the Affera Mapping and Ablation system CE Mark, demonstrating that the Sphere-9 catheter can successfully treat...
Medtronic receives CE Mark approval for Affera mapping and ablation system...
Medtronic has announced today that it has received CE Mark for the Affera mapping and ablation system, which includes the Sphere-9 catheter and the...
Healthy or hazardous: Study raises “red flag” on wearable smart device...
A new “red flag-raising” study has provided benchmark data on the safety of smart scales, watches and rings with bioimpedance technology for patients with...
Medtronic receives CE mark for EV-ICD device to treat “dangerous” heart...
Medtronic has received CE mark for the Aurora extravascular implantable cardioverter-defibrillator (EV-ICD) magnetic resonance imaging (MRI) SureScan device and Epsila EV MRI SureScan defibrillation...
Medtronic completes enrolment in pulsed field ablation catheter trial for patients...
Medtronic has announced enrolment completion and final treatment in the SPHERE Per-AF, a US Food and Drug Administration (FDA) Investigational Device Exemption (IDE) trial....
Medtronic adds cardiac mapping platform through Affera acquisition
Medtronic has announced that it has completed the acquisition of Affera. The acquisition will expand Medtronic’s cardiac ablation portfolio to include its first cardiac...
Medtronic names Laura Mauri as new chief scientific, medical and regulatory...
Medtronic has announced that Laura Mauri has been appointed as the company’s chief scientific, medical and regulatory officer. This appointment adds to Mauri's prior responsibilities...
Medtronic to acquire Affera
Medtronic has announced that it has entered into an agreement to acquire medical technology company Affera.
Affera designs and manufactures cardiac mapping and navigation systems...
US and European approval for Medtronic’s LINQ II insertable cardiac monitor
Medtronic today announced that it has received US Food and Drug Administration (FDA) clearance and CE mark approval for its LINQ II insertable cardiac...
Medtronic’s Micra AV TPS receives CE mark
Medtronic has received CE mark for Micra AV Transcatheter Pacing System (TPS), the world’s smallest pacemaker with atrioventricular (AV) synchrony.
Micra AV is indicated for...
Medtronic shares results of BlueSync and Micra TPS CED studies
Medtronic has announced results from late breaking clinical trials, presented online at HRS 2020 Science, evaluating the MyCareLink Heart mobile app and the Micra...
Results of PULSED AF trial presented at HRS 2020 Science
Initial results of the PULSED AF trial, the first-in-human results for paroxysmal or persistent atrial fibrillation (AF) treated with pulsed field ablation (PFA) were...
Medtronic provides ventilator progress update
Medtronic has announced updates regarding its efforts to increase ventilator production around the globe. The company is announcing progress in the ramp-up of its...
Medtronic shares ventilation design specifications to accelerate efforts to increase global...
Medtronic has announced it is publicly sharing the design specifications for the Puritan Bennett 560 (PB 560) to enable participants across industries to evaluate...
AF Symposium 2020: Cryoablation pulmonary vein isolation is safe and effective...
Hugh Calkins (Johns Hopkins Medical Institutions, Baltimore, USA) told delegates attending the AF Symposium 2020 (23–25 January, Washington, DC, USA) that cryoablation with the...
AF Symposium 2020: Myocardial specificity of pulsed field ablation provides safety...
At the AF Symposium 2020 (23–25 January, Washington, DC, USA), Vivek Y Reddy (Helmsley Electrophysiology Center, Icahn School of Medicine at Mount Sinai, New York, USA)...
Medtronic receives FDA approval for Micra AV
Medtronic has announced that it has received US Food and Drug Administration (FDA) approval of Micra AV, the world’s smallest pacemaker with atrioventricular (AV)...
Omar Ishrak to step down as Medtronic CEO next year
Omar Ishrak, Medtronic’s chairman and CEO is to retire on 26 April 2020, following the end of the company’s current fiscal year. Also, the...
New catheter system for His bundle pacing receives US FDA clearance
Medtronic has announced US Food and Drug Administration (FDA) clearance and commercial launch for the SelectSite C304-HIS deflectable catheter system for use in procedures...
Targeted blood pressure management helps to reduce strokes in patients with...
An analysis on the impact of stroke severity in patients receiving the HeartWare HVAD system (Medtronic) as destination therapy shows that targeted blood pressure...
Medtronic to acquire EPIX Therapeutics
Medtronic has announced that it has entered into a definitive agreement to acquire EPIX Therapeutics. When completed, the EPIX acquisition will expand the Medtronic...
First patient treated in TERMINATE AF clinical trial
The first patient has been treated in the TERMINATE AF trial (Medtronic). This is a multi-centre study evaluating two surgical ablation devices—the Cardioblate irrigated...
Medtronic disables internet updates in CareLink CIEDs due to cybersecurity risks
Medtronic has issued a software update "to address a safety risk caused by cybersecurity vulnerabilities" in 34,000 of their cardiac implantable electronic devices (CIEDs),...
Study shows improved quality of life and reduced symptoms in patients...
New findings from the CRYO4PERSISTENT AF clinical trial demonstrate improved quality of life, reduced symptoms from abnormal heart rhythms, and low incidence of reinterventions...
Clinical trial to evaluate ECG belt for optimising heart failure therapy...
Medtronic has announced the first enrolments in a new clinical trial evaluating the ECG (electrocardiography) Belt Research System (ECG Belt) as a diagnostic tool...
Expanded indication for pacing lead for His bundle site stimulation
A press release has announced US Food and Drug Administration (FDA) labeling expansion for the Medtronic SelectSecure MRI SureScan Model 3830 cardiac pacing lead...
FDA classifies HeartWare HVAD systems unexpected power source switching as Class...
The United States Food and Drug Administration (FDA) has classified Medtronic recent voluntary urgent field action related to the HeartWare HVAD System unexpected power...
Study confirms feasibility of new extravascular approach to ICD therapy
Results from a research study demonstrating the feasibility of a novel approach to delivering pacing and defibrillation therapy in which a lead is placed...
Medtronic: class I recall- manufacturing error preventing electrical shock delivery
Medtronic is recalling certain implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators (CRT-Ds) due to a defect in the manufacturing process. This defect...
Medtronic HeartWare HVAD approved for destination therapy
The US Food and Drug Administration (FDA) has approved Medtronic’s HeartWare HVAD system as a destination therapy for patients with advanced heart failure who...
CE mark granted to Medtronic for Attain Stability Quad MRI SureScan...
Medtronic has received CE mark for the Attain Stability Quad MRI SureScan left heart lead. The device offers active-fixation technology, designed for precise lead...
First patients enrolled in Medtronic’s STOP AF First cryoballoon clinical trial
The first participants have been enrolled in Medtronic’s STOP AF First clinical trial. The trial will evaluate the safety and effectiveness of performing pulmonary...
Improved therapy and reduced healthcare costs demonstrated for Medtronic CRT devices
New data showing the use of Medtronic’s cardiac resynchronisation therapy (CRT) devices—with its proprietary AdaptivCRT and EffectiveCRT algorithms—to result in lower healthcare system costs...
Medtronic Reactive ATP therapy slows atrial fibrillation progression in real-world population
Medtronic has announced that its Reactive atrial-based antitachycardia pacing (ATP) therapy slows the progression of atrial fibrillation (AF) in patients with implanted cardiac devices....
Data reinforce benefits of Medtronic cardiac resynchronisation therapy technologies in ‘real-world’...
New data supporting the clinical performance of the company's exclusive EffectivCRT diagnostic and AdaptivCRT algorithm in heart failure patients who receive cardiac resynchronisation therapy...
HRS 2017: True incidence of atrial fibrillation is underestimated
A substantial number of patients at risk for atrial fibrillation (AF) may remain undetected using conventional monitoring techniques, a late-breaking clinical trial session at...
Medtronic receives FDA approval for MR-conditional quadripolar CRT pacemakers
Medtronic has received US Food and Drug Administration (FDA) approval for a portfolio of quadripolar cardiac resynchronization therapy-pacemakers (CRT-Ps) that are designed to improve...
Medtronic ENDURANCE Supplemental trial of HVAD fails to meet primary endpoint
Medtronic’s ENDURANCE Supplemental trial of the company’s HVAD ventricular assist device has failed to meet its primary endpoint of all neurological events at 12...
Medtronic initiates global trial of Arctic Front Advance cardiac cryoablation catheter
Medtronic has enrolled the first participants in the STOP Persistent AF clinical trial. The trial will evaluate the safety and effectiveness of a pulmonary...
US FDA approves expanded indication for Medtronic Freezor Xtra cryoablation catheter
The US Food and Drug Administration (FDA) has approved Medtronic’s Freezor Xtra cryoablation catheter for treating patients with atrioventricular nodal re-entrant tachycardia (AVNRT). The...
Advancements in Persistent AF Ablation: New Clinical Evidence and Techniques
This webinar has been sponsored by Medtronic.
Watch the recent webinar on persistent Atrial Fibrillation (AF) ablation with Prof. Schilling (St. Barts, London), Prof. de...
US FDA clears CardioInsight noninvasive 3D mapping system
Medtronic has received US Food and Drug Administration (FDA) 510(k) clearance for the CardioInsight noninvasive 3D mapping system. The CardioInsight system is used to...
Discover the latest evidence and ablation techniques for persistent AF treatment
Join atrial fibrillation (AF) experts Prof Richard Schilling (St Barts Hospital, London UK), Prof Carlo de Asmundis (Universitair Ziekenhuis, Brussels, Belgium) and PD Dr...
Medtronic receives CE mark for less-invasive HVAD implant procedure
Medtronic’s HVAD system left ventricular assist device (LVAD) has received CE mark for a less-invasive implant procedure in patients with advanced heart failure. The...
US FDA approves Medtronic’s Claria MRI Quad cardiac resynchronisation therapy defibrillator
Medtronic has received US Food and Drug Administration (FDA) approval for the Claria MRI Quad cardiac resynchronisation therapy defibrillator (CRT-D) SureScan device for patients...
Medtronic gets FDA approval for MRI compatible cardiac rhythm and heart...
Medtronic has announced US Food and Drug Administration (FDA) approval for its suite of cardiac rhythm and heart failure devices and leads to be...
ESC 2016 Clinical Practice Guidelines describe benefits of cryoballoon ablation and...
Cryoballoon ablation for patients with diagnosed atrial fibrillation, and long-term cardiac monitoring for survivors of stroke who do not have an established diagnosis of atrial...
New alliance for heart failure calls for improved care for condition
A coalition of charities, patient groups, professional bodies and healthcare companies with an interest in heart failure has been established to raise the profile...
Medtronic EffectivCRT-during atrial fibrillation algorithm can improve therapy delivery
The Cardiac Resynchronization Therapy Efficacy Enhancement (CRTee) study has shown that the Medtronic EffectivCRT-during atrial fibrillation algorithm can improve therapy delivery in heart failure...
Medtronic Micra TPS sustains low complication rate through 12 months
New long-term results from the Medtronic Micra Transcatheter System Global Clinical Trial have shown consistently low rates of major complication. The data—from largest and...