The American College of Cardiology (ACC) has released first findings from its expanded outpatient registry, the PINNACLE registry, focusing on atrial fibrillation and including the next generation of anticoagulants.
The Nicom (Cheetah Medical) haemodynamic monitoring system provides greater insight on patient status in the trauma setting and leads to a significant decrease in patients' length of hospital stay.
The recommendation is due to an increase in frequency of reported premature Riata insulation failures.
The Lumax 740 defibrillator (Biotronik) differentiates atrial from ventricular arrhythmias to avoid unnecessary shocks.
This service is a remote monitoring system that enables clinicians in healthcare facilities to quickly obtain data regarding the status of Medtronic implanted cardiac devices, facilitating faster treatment decisions.
The new Carto 3 Multi-Electrode Mapping (MEM) version provides a fast method for mapping and diagnosing arrhythmias, while using sensor-based MEM-enabled catheters to maintain a high degree of anatomical accuracy.
Gregory Lip and colleagues have made an indirect comparison of the new anticoagulants. They have found "no profound significant differences" in efficacy between apixaban (Eliquis, Pfizer and Bristol-Myers Squibb) and both does of dabigatran (Pradaxa, Boehringer Ingelheim) and Rivaroxaban (Xarelto, Bayer).
A preliminary study, by Robert Hauser and others, published in EP Europace, indicates that a silicone-polyurethane copolymer (Optim, St Jude Medical) designed to prevent implantable cardioverter defibrillator (ICD) lead insulation abrasions fails to prevent such abrasions.
In a joint consensus statement, the Heart Rhythm Society (HRS) and the American College of Cardiology Foundation (ACCF) have issued recommendations, in collaboration with the Society of Thoracic Surgeons, on the use of single- vs. dual-chamber devices for patients who are eligible (according to existing guidelines) for pacemaker implantation.
Researchers in The Netherlands have found that the use of implantable cardioverter defibrillators (ICDs) may be partly responsible for the reduction in the number of ventricular fibrillation out of hospital cardiac arrests.
The total consideration to be paid by CardioNet will be US$23.5 million. The transaction, subject to customary closing conditions, is expected to close in the third quarter of 2012.
With the Image Ready technology, Ingenio MRI pacemakers, in combination with Fineline II leads, allow patients to undergo MRI procedures as needed.
International consensus writing group provides specific recommendations on mode choices for single- versus dual- chamber devices for adult population.
The aim of the study is to assess the economic effects of Biotronik Home Monitoring based remote follow-up management compared to conventional in-clinic or practice follow-ups.
The Unify Quadra CRT-D system was designed to help the heart perform in its most natural state by synchronising the left and right ventricles of the heart through timed electrical pulses.
Initial phase-one results of the St Jude Medical's Riata Lead Evaluation study have found that externalised conductors occurred in 9.3% of the smaller-diameter Riata ST 7F leads, and in 24% of the larger-diameter Riata 8F leads.
CardioFit is an implantable electrical stimulation device designed to improve heart function in patients with congestive heart failure.
ViewFlex Xtra Intracardiac Echocardiography catheter (St Jude Medical) is used to treat arrhythmias with radiofrequency ablation and to close atrial septal defects with structural heart procedures.
The SPIRIT-ICD study explores benefits of intensified diagnostic follow-up and timely therapeutic intervention for high-risk patient group receiving ICD therapy.
Data from the MADIT-CRT study indicates that there are six baseline factors that predict left ventricular ejection fraction (LVEF) "super response" in patients with a cardiac resynchronisation therapy with defibrillator (CRT-D) device, report Jonathan Hsu and colleagues.
The hybrid procedure, which combines a transvenous endocardial and a thoracoscopic epicardial approach in a single ablation procedure, is associated with a 83% success rate at one year in patients with atrial fibrillation, a study has found.
The Sudden Cardiac Arrest Patient Education DVD is the Society’s first-ever educational DVD developed as a resource specifically for patients with SCA and part of the Society’s annual SCA awareness initiatives. The DVD uses a mix of real-world video...
California Medical Laboratories develop, manufacture and distribute a complete range of cannulae, catheters and accessories for cardiac surgery.
According to the company, this is the world's first portable echocardiography system to offer real time 3D Transesophageal Echo (Live 3D TEE), which enables 3D visualisation of the cardiac structure and function as well as new perspectives of the heart in real time.
A new report launched on 3 July written by UK experts shows that atrial fibrillation, a condition that affects up to 1 million people in the UK, is not being optimally managed.
The Sudden Cardiac Arrest Patient Education DVD is the Society's first-ever educational DVD developed as a resource specifically for patients at risk of sudden cardiac arrest and part of the Society's annual SCA awareness initiatives.
The PREVAIL study is designed to gain FDA approval for the Watchman Left Atrial Appendage (LAA) Closure device (Boston Scientific).
Results from a recently completed clinical study suggest that a 32mg single intravenous dose of ondansetron (Zofran, GlaxoSmithKline) may affect the electrical activity of the heart, which could pre-dispose patients to develop Torsades de Pointes.
Pia Schuler, The Grown Up Congenital Heart Disease Unit, The Heart Hospital, University College London Hospitals, London, UK, and others reported that there is little data for the effect of pregnancy on women with ICDs. Thus, they reviewed the outcome of pregnant women with an ICD at their centre.
St Jude Medical states: Through our investigation of the MAUDE database report submitted to the FDA on 2 May 2012, and information provided to the company by the FDA, including model number, implant and event dates, St Jude Medical has identified a single Durata lead that matches the available information.
Bristol-Myers Squibb and Pfizer announced that the FDA has issued a complete response letter regarding the new drug application for apixaban (Eliquis) for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation.
A study published in the Archives of Internal Medicine indicates that in the long-term, rhythm control for atrial fibrillation may be associated with lower mortality than rate control.
Stefan Kääb outlines the different steps to developing comprehensive personalised medicine strategies in the context of cardiac arrhythmias and reviews what essential elements such strategies require.
A study, which suggested that a novel therapy for managing cardiovascular implantable electronic device (CIED) infections could markedly diminish the need for device removal, sparked a lively discussion at Cardiostim (13-16 June, Nice, France).
According to a study presented by Laurent Roten, Bordeaux, France, at Cardiostim, in patients with early repolarisation and who had survived a sudden cardiac arrest, global and inferior J wave amplitudes were significantly higher in patients with recurrent ventricular fibrillation than in the remaining patients.
Scott Beau and colleagues outlined a new implantable cardioverter defibrillator (ICD) algorithm that could be used to discriminate ventricular tachycardia/ventricular fibrillation noise from pace/sense conducted lead noise, potentially reducing inappropriate therapy due to lead noise.
Unilect, Neutralect, and Biotab electrodes are now available alongside Ambu Blue Sensor and Ambu Neuroline.
John Camm and Karl-Heinz Kuck, on behalf of the European Heart Rhythm Association (EHRA), review the recent consensus document from the Royal College of Physicians of Edinburgh (RCPE) on the management of atrial fibrillation.
Short-term antiarrhythmic drug treatment after cardioversion is nearly as effective in preventing recurrences of atrial fibrillation as long-term treatment, data from Flec-SL show.
Chu-Pak Lau, honorary clinical professor, Division of Cardiology, Department of Medicine, University of Hong Kong, Queen Mary Hospital, Hong Kong, talked to Cardiac Rhythm News about rate-responsive pacemakers, the potential role of gene therapy in managing atrial fibrillation, and leadless pacing.
Using the Smartview remote monitoring solution, healthcare providers can now access valuable cardiac data and alert messages from Sorin's implantable Paradym RF devices, while the patient is at home.
Endosense and Biotronik have announced that Endosense's TactiCath Quartz force-sensing ablation catheter has been CE mark approved in Europe.
The European Heart Rhythm Association (EHRA) a registered branch of the European Society of Cardiology (ESC) and Cardiostim"Reed have agreed to hold a joint annual congress during 2014"2017.
The system offers wireless and automatic remote follow-up and optional integrated cellular GSM connectivity.
The session will include electrophysiology-related presentations made by leading female physicians and researchers from around the world. Topics will include "Atrial fibrillation ablation: beyond pulmonary vein isolation" and "Controversies in lead extractions for noninfectious indications."
Royal Philips Electronics has launched the Save Lives website (www.SaveLives.net), an online campaign to inform people about sudden cardiac arrest and to empower them to act.
https://youtu.be/EbQ0FJLuL2o
The Save Lives website is an online campaign to inform people about sudden cardiac arrest and to empower them to act.
Boston Scientific has added the first and only, commercially available, subcutaneous implantable cardioverter defibrillator technology to its product portfolio
Zoll, manufacturer of the LifeVest wearable defibrillator and the RecueNet Medgate 12-Lead management solution, will display its products at Cardiostim (13-16 June, Nice, France) this week
At a median follow-up time of 22 months, patients with an abnormal MTWA test were 11 times more likely to experience a major arrhythmic cardiac event than patients with a normal MTWA result.
Karl-Heinz Kuck is currently director of Cardiology at the Asklepios Klinik St Georg in Hamburg, Germany. He has authored more than 450 scientific papers, has served as a board member of the European Heart Rhythm Association (EHRA) for the past four years and is the current EHRA president-elect.
An article in the Journal of Cardiovascular Electrophysiology outlines the development and results of the new interdisciplinary procedure, the convergent procedure
Yoram Rudy, St Louis, USA, talked to Cardiac Rhythm News about the novel imaging technique, electrocardiographic imaging, which was developed in his laboratory.
Mills replaces Fred A Middleton, who will remain on the board as an independent director.
A Finish study has found that men whose electrical impulses take a few milliseconds longer to travel through the lower chambers of the heart had an increased risk of sudden cardiac death.
The guidance recommends rivaroxaban for people with non-valvular atrial fibrillation who have one or more risk factors such as congestive heart failure, hypertension, age 75 years or older, diabetes mellitus, prior stroke or transient ischaemic attack.
A study presented by Rachel Lampert, New Haven, USA, at HeartRhythm 2012 (9-12 May, Boston, USA) shows that sports participation of patients with an implantable cardioverter defibrillator (ICD), in many athletes, is not associated with any serious adverse events.
Lead author Martin Burke, University of Chicago Hospitals, USA, presented a study at HeartRhythm 2012 that showed the subcutaneous implantable cardioverter defibrillator (S-ICD) to be an effective treatment for patients with ventricular tachyarrhythmias.
Data presented at HeartRhythm 2012 on the ADVANCE III trial have shown that a greater number of intervals to detect arrhythmias significantly reduces unnecessary implantable cardioverter defibrillator (ICD) therapy.
nContact has announced that new long-term physician data demonstrated promising clinical outcomes for the multidisciplinary Convergent Procedure in the treatment of persistent atrial fibrillation (AF), with over 80% of patients remaining in sinus rhythm at a minimum of 12 months follow-up.
A large cohort study from Norway has found that the strongest risk factors for atrial fibrillation in both men and women were a history of palpitations and hypertension.
The announcement was made at the Presidents' Reception on Friday 11 May. Gillis now serves as the 34th president of HRS and the second international president preceded by Bernard S Goldman, (1982-1983).
An economic analysis of the RAFT trial showed a US$33,025 cost per Quality Adjusted Life Year (QALY) gained using Medtronic CRT-Ds in a mild, New York Heart Association (NYHA)-designated Class II-III heart failure patient population.
Vivek Reddy, New York, USA, presented results from the ASA Plavix (ASAP) study of the Watchman Left Atrial Appendage Closure (LAAC) device (Boston Scientific) during a late-breaking session at the Heart Rhythm Society's 33rd Annual Scientific Sessions in Boston, USA.
Boston Scientific has announced extended warranties of up to 10 years, depending on the model, for its Energen and Incepta implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators (CRT-Ds).
ESCAPE-ICD is a large-scale observational study, sponsored by Biotronik, designed to tackle the alarming lack of data on sudden cardiac death in Latin America.
Electrophysiology thought leaders gathered to discuss the significance of posterior ablation in the treatment of arrhythmias, with presentations highlighting the importance of isolating the posterior left atrium with an adjunctive epicardial treatment.
Martin C Burke, University of Chicago, USA, presented the results of the S-ICD System investigational device exemption clinical trial at a late-breaking session at the Heart Rhythm Society's 33rd Annual Scientific Sessions (9-12 May, Boston, USA).
The Thermocool SF NAV catheter is designed to reduce fluoroscopy exposure to physicians, lab staff and patients alike, enhance visualisation of the ablation catheters orientation and improve procedural efficiency.
The Ellipse implantable cardioverter defibrillator (St Jude Medical) is the industry's smallest high-energy ICD and it has been designed with feedback from more than 200 physicians from around the world
Biotronik will showcase the Lumax 740 ICD and CRT-D series and the Evia HF-T with ProMRI, a technology that allows patients requiring tachycardia and heart failure therapies to have access to magnetic resonance imaging.
CardioFocus has announced that its HeartLight Endoscopic Ablation System (EAS) for the treatment of atrial fibrillation will be highlighted both as part of the scientific sessions and during a private academic symposium at the Heart Rhythm Society's 33rd Annual Scientific Sessions (9-12 May, Boston, USA).
The FDA has approved the Ingenio and Advantio pacemakers and Invive cardiac resynchronisation therapy pacemakers (CRT-P).
Stereotaxis has announced that a total of 11 invited podium presentations will feature the use of Stereotaxis magnetic navigation technology in a wide variety of arrhythmias.
The procedure, known as left atrial appendage occlusion, is performed using the FDA-approved Lariat Suture Delivery Device (SentreHeart).
Initial findings from the "Dabigatran in the real world" study reveal that complications necessitating therapy discontinuation occurred more frequently with dabigatran than with warfarin.
Design and technology consultancy Cambridge Design Partnership has announced that it has completed a research project to identify the future of medical technology to treat atrial fibrillation.
The Heart Rhythm Society (HRS) has launched a Boston citywide awareness campaign to increase knowledge about symptoms, warning signs and available treatment options for atrial fibrillation and sudden cardiac arrest.
Evia HF-T is the first cardiac resynchronisation therapy (CRT-P) device approved for use in an MRI environment.
GlideLight requires 55% less force to advance in the removal of cardiac leads than the existent SLS II laser sheath (Spectranetics).
After the international launch on 17 April 2012, the first device implantation of a Lumax 740 ICD in Asia was successfully performed by Jeffrey Wing-Hong Fung, director of Electrophysiology Laboratory at Hong Kong Adventist Hospital.
The long-life battery provides for eight hours of continuous operation to monitor and document a patient's ECG during a flight, and to provide defibrillation shocks as required.
Some patients with certain types of atrial fibrillation who are taken off anti-clotting medication face a high risk of stroke or blood clotting within a month, according to new research presented at the American Heart Association's Emerging Science Series webinar.
A new study, published in Circulation: Arrhythmia and Electrophysiology, indicates that catheter ablation of ventricular tachycardia is an important treatment option in patients with ARVD/C, despite a high rate of ventricular tachycardia recurrence, because it reduces the burden of the arrhythmia
A study published in the Journal of Cardiovascular Electrophysiology shows that catheter ablation of octogenarians with atrial fibrillation is as safe and effective as catheter ablation of atrial fibrillation in younger patients
The registry programme will collect important data on the safety and comparative effectiveness of antithrombotic treatments, including vitamin K antagonist warfarin, acetylsalicylic acid and novel oral anticoagulants, such as dabigatran etexilate.
The medical device manufacturer St Jude Medical has called for the Heart Rhythm Journal to retract an online study in which the company's recalled Riata and Riata ST implantable cardioverter defibrillator (ICD) leads were associated with 71 deaths and Medtronic's Quattro Secure lead was associated with 62 deaths.
Elsayed Soliman, Winston-Salem, USA, and colleagues have found that prolonged QTc is associated with a significantly increased risk of stroke, indicating that drugs that prolong QTc may need to be investigated for a potential association with stroke.
Hugh Calkins, chair of the upcoming Heart Rhythm 2012─Scientific Sessions Program Committee, spoke to Cardiac Rhythm News about his career highlights, Heart Rhythm 2012, and the new consensus document on catheter and surgical ablation of atrial fibrillation.
Rachel Lampert, New Haven, USA, is giving the talk "When to consider ICD deactivation: End-of-life issues" at Heart Rhythm 2012 (9-12 May 2012, Boston, USA). She talked to Cardiac Rhythm News about this topic.
Positive clinical data support Synergy's potential to improve haemodynamics, exercise capacity and quality of life in INTERMACS 4, 5, and 6 patients with chronic heart failure.
The Ellipse (St Jude Medical) ICD's design was conceptualised by more than 200 physicians during focus groups in which they crafted in clay their vision for the ideal device design.
The Medtronic Foundation's HeartRescue Project has produced an interactive, online experience, the "Save-a-Life Simulator," to promote proper and timely bystander response to sudden cardiac arrest (SCA).
The Ingenio and Advantio pacemakers feature RightRate pacing technology designed to treat chronotropic incompetence.
The FDA's decision was supported with data from the pivotal REVERSE and landmark RAFT clinical trials, which showed that CRT-D can benefit mildly symptomatic heart failure patients by reducing mortality and heart failure hospitalisation rates.
The REPLACE study is the first prospective, multicentre trial to examine a broad range of complications related to replacement of all companies' cardiovascular implantable electronic devices (CIED) which includes both pacemakers and ICDs (implantable cardiac defibrillators).
The RECORD investigation of CLS pacemakers is the largest to date and demonstrates excellent performance in all pacing sites within the right ventricle (apical, septal, outflow tract), and even in advanced heart failure with no difference to patients without heart failure.
DigiFab (BTG) is indicated for the treatment of patients with known (or strongly suspected) life-threatening digoxin toxicity associated with ventricular arrhythmias or bradyarrhythmias.
If oral anticoagulation therapy is indicated in patients with atrial fibrillation, most patients should receive one of the three new anticoagulants ─ dabigatran, apixaban or rivaroxaban ─ (once approved) rather than warfarin, according to guidelines published in the Canadian Journal of Cardiology.
A study published in the Journal of the American Medical Association has found that an invasive strategy of systematic electrophysiology study and prophylactic pacemaker implantation in patients with myotonic dystrophy type one and major infranodal conduction delays was associated with a higher rate of nine-year survival than a non-invasive strategy.
Results from the ISSUE 3 study show that active pacemaker therapy can reduce episodes of syncope in selected patients with severe asystolic neurally mediated syncope. Data were presented by Michele Brignole at the American College of Cardiology conference.
Following its recent recommendation for dabigatran (Pradaxa, Boehringer Ingelheim), the UK's National Institute for Health and Clinical Excellence (NICE) now recommends rivaroxaban (Xarelto, Bayer) as an option for preventing stroke and systemtic embolism in patients with non-valvular atrial fibrillation.
In a study presented by Janez Toplisek, at ACC 2012, 85% of patients who underwent ablation with the multidisciplinary Convergent Procedure were in sinus rhythm at one year follow-up, had a significant reduction in left atrial size and improvement in left ventricular ejection fraction.
The guidance outlines clinical data, risks, benefits and patient risk tolerance.
The AliveCor smartphone ECG is an innovative, investigational medical wireless device which incorporates electrodes into a wireless case that snaps onto the back of a smart phone, allowing for wireless single-lead recording of 30-second rhythm strips that are stored securely in the cloud and the device itself.
PHRI will review data from three combined registries on the Riata ST Optim and Durata implantable cardioverter defibrillator (ICD) leads.
Physicians at Isala Klinieken in Zwolle, Netherlands, were the first in the country to incorporate CardioFocus' visually guided ablation technology.
The new pacemaker and lead allow patients to undergo full-body, high-resolution magnetic resonance imaging (MRI) scans.
A study, published ahead of print in Circulation Cardiovascular Quality Outcomes, shows that the Data Extraction and Longitudinal Trend Analysis (DELTA, Coping Systems) system could have triggered a sustained safety alert about the Sprint Fidelis lead (Medtronic) two years before the product was taken off the market.
Amitava Banerjee, Birmingham, UK, and co-authors have used "real world" data to show that three new oral anticoagulants have greater net clinical benefit than warfarin in patients with atrial fibrillation who have a high risk of stroke and bleeding.
Results from the TARGET study have shown that using speckle-tracking echocardiography to target left ventricular lead placement for cardiac resynchronisation therapy (CRT) significantly improves the rate of response and clinical status compared with conventional therapy.
The latest innovations of the HeartStart MRx are based on extensive feedback from emergency medical services professionals.
A software monitoring program that tracks implantable cardioverter-defibrillator (ICD) function could detect devices' malfunctions earlier than current monitoring processes, according to new research published in Circulation: Cardiovascular Quality and Outcomes.
Cameron Health is the developer of the world's first and only commercially available subcutaneous implantable cardioverter defibrillator - the S-ICD system.
The CapSure Sense MRI SureScan pacing leads (Medtronic) will give physicians an additional option that is MR-conditional, or designed to be safe for the MRI environment when used per specified MR conditions.
The first peer-reviewed video demonstrating an implantable cardioverter-defibrillator (ICD) implantation has been published in JoVE, the Journal of Visualized Experiments.
Bristol-Myers Squibb and Pfizer have announced that the US Food and Drug Administration (FDA) has extended the action date by three months for the new drug application for apixaban (Eliquis) for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
According to a study published in the February issue of the Canadian Medical Association Journal, cognitive and functional decline are "important consequences" of atrial fibrillation even in the absence of overt stroke.
ATTEST, a prospective, multicentre, randomised, controlled study will compare pulmonary vein isolation (using Biosense Webster's ThermoCool catheters and the company's Carto 3 or Carto XP mapping systems) with drug therapy (rate or rhythm control) in patients with paroxysmal atrial fibrillation.
Eline Nannenberg, Amsterdam, The Netherlands, and colleagues have found that the Family Tree Mortality Ratio (FTMR) method can identify the age ranges that carry the greatest risk of death from an inherited arrhythmia syndrome.
A study published online in the HeartRhythm Journal indicates that while the sodium channel blocker test has good prognostic value in symptomatic patients with non-diagnostic Brugada ECG (Br-ECG), it appears to have little value in asymptomatic patients.
These labs feature technologies across the cardiovascular service line for hospitals and clinics interested in developing state-of-the-art laboratories that meet their changing clinical needs.
A new preclinical study shows that a unique minimally invasive percutaneous approach to accessing the heart may enable electrophysiologists to perform epicardial ablation for ventricular tachycardia.
The HeartStart XL+ is the only Philips defibrillator that, in AED mode, can defibrillate any patient - adult or child - with no special pads required.
The Teligen implantable cardioverter defibrillators (Boston Scientific), designed to treat sudden cardiac death, are the smallest high-energy devices available in China.
The HeartLight EAS US pivotal trial is designed to evaluate the safety and efficacy of the HeartLight Endoscopic Ablation System (EAS) (CardioFocus) in treating symptomatic atrial fibrillation by creating electrical isolation of the pulmonary veins.
The anticoagulant rivaroxaban (Bayer HealthCare, Xarelto) has been accepted for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation who have one or more risk factors and for the treatment of deep vein thrombosis (DVT) and prevention of recurrent DVT and pulmonary embolism, following an acute DVT in adults.
The xCELLigence Cardio Instrument is an impedance sensing instrument capable of reading signals at high sampling rates, making it possible to measure the contraction movements of cardiomyocytes in contact with sensor microelectrodes.
The Vdrive system (Stereotaxis) allows physicians to remotely manipulate traditionally non-robotic catheters.
EP Europace becomes the official journal to the European Heart Rhythm Association (EHRA), the European Society of Cardiology (ESC) Working Group (WG) on Cardiac Cellular Electrophysiology, and the ESC Working Group on e-Cardiology. It will additionally invite articles on research topics into the application of computing technology and data processing for electrophysiology.
In an editorial in the New England Medical Journal, three leading specialists in the management of cardiac rhythm disorders argue for a more "considered and nuanced" approach to replacing implantable cardioverter defibrillators (ICD).
By Manuel Castella
Since time that the FAST trial1 was designed in 2007, up to now, there has not been clear evidence to indicate ablation treatments aside from the control of symptoms.2,3 Nevertheless, catheter ablation has expanded its indications thanks...
The version 5.0 of the Merlin.net Patient Care Network includes a Mobile DirectAlerts notification feature for important patient and device alerts. This is a secure physician notification system compatible with iPhone, Android, iPad and Blackberry.
A sub-analysis of data from the ROCKET-AF trial presented at the American Stroke Association's International Stroke Conference 2012 shows that rivaroxaban (Xarelto, Bayer Healthcare) might be better at preventing clot-related strokes in patients with atrial fibrillation while minimising the risk of causing a bleeding stroke.
Occasional erratic heart rhythms appear to cause about one-fifth of strokes for which a cause is not readily established, according to research presented at the American Stroke Association's International Stroke Conference 2012.
2D Cardiac Performance Analysis MR analyses myocardial function and deformation in standard magnetic resonance cine sequences and does not require specific acquisition modes like tagged imaging.
The DF4 (Medtronic) is a right ventricular lead and connector used with implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators (CRT-Ds) to detect and accurately treat potentially life-threatening heart rhythms.
The US National Institutes of Health 2.5-year grant will help test a miniature, battery-free, wireless device that will reduce the need for invasive cardiac catheterisation procedures and provide a better understanding of congenital heart disease in infants and children.
Middle-aged men at the upper end of normal blood pressure had an elevated risk for atrial fibrillation later in life, according to new research published in Hypertension: Journal of the American Heart Association.
Biotronik announced it has successfully completed its final interoperability testing process to connect Biotronik Home Monitoring to electronic health record systems through the Biotronik EHR DataSync technology.
Angelo Auricchio, president of the European Heart Rhythm Association, reviews the highlights of his impressive career with Cardiac Rhythm News.
Hugh Calkins, Maryland, USA gave a sneak preview of the forthcoming 2012 document on catheter ablation for atrial fibrillation. At Boston AF he said, the new document is not just a "brief update" of the previous HRS/EHRA/ECAS document, which was published in 2007, but a "comprehensive, state-of-the-art review of the field of catheter and surgical ablation for atrial fibrillation."
At Boston AF, Peter Kowey, Pennsylvania, USA, declared that the recent failure of the PALLAS study did not mean that they were "throwing in the towel" with dronedarone and announced plans for a new dronedarone study.
A new study, published in the New England Journal of Medicine, has added weight to the view that subclinical atrial fibrillation is a possible cause of cryptogenic stroke after finding that 13% of strokes and systemic embolisms in the study population were associated with subclinical atrial tachyarrhythmias.
At Boston AF, two poster presentations showed that a multidisciplinary procedure that uses both epicardial and endocardial ablation (convergent procedure) is associated with high single procedure efficacy in a patient population dominated by persistent atrial fibrillation.
Fire and Ice is a prospective, randomised, multinational head-to-head clinical trial comparing the long-term safety, effectiveness and ease of use of the Medtronic Arctic Front cardiac cryoablation system compared to the Biosense Webster Carto system guided Thermocool catheter to treat patients with symptomatic paroxysmal atrial fibrillation.
This new centre of excellence, selected by Hansen Medical, will serve as the training centre for the Northern United States and is expected to commence operation in February 2012.
A new report by visiongain predicts that the overall world antithrombotic and anticoagulant drug market will reach US$24.4 billion in 2015.
Researchers in Germany achieve 98% acute pulmonary vein isolation, with 77% of patients in sinus rhythm at approximately nine months follow-up.
The TigerPaw system II (LAAx) is indicated for occlusion of the left atrial appendage under direct visualisation, in conjunction with other open cardiac procedures.
The registry will gather information from valvular and non-valvular atrial fibrillation patients. It will also track the impact of new anticoagulant therapies on stroke prevention, in addition to collating insights into patients' satisfaction with their entire atrial fibrillation management and the overall health economic burden of atrial fibrillation across Europe.
The new pacemaker and lead allow patients to undergo full-body, high-resolution magnetic resonance imaging (MRI) scans.
Study illustrates the effectiveness of detecting patients with higher risk of stroke using implantable device monitoring.
Preview of the revised HRS/EHRA/ECAS consensus document on catheter ablation for atrial fibrillation at Boston AF (12-14 January 2012, Boston, USA) reveals new recommendation to use the CHADS2-VASc score
The Thermocool SF irrigated ablation catheter (Biosense Webster) has been approved for treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation when used with Carto systems and type 1 atrial flutter for patients 18 years and older.
The TactiCath 75 is an 8.5 F sheath compatible, state-of-the-art open irrigated, steerable radiofrequency ablation catheter that employs the same proprietary force-sensing technology as the original TactiCath but features a longer, 75 millimeter curved tip.
The SMART-AF clinical study is designed to demonstrate the safety and effectiveness of the Thermocool Smarttouch catheter (Biosense Webster) in the treatment of symptomatic, drug-refractory, paroxysmal atrial fibrillation.
The first day of Boston AF (12 January 2012, Boston, Massachusetts, USA) was a mixture of best paper reviews and live case presentations.
The Montreal Heart Institute will work with GnuBIO to develop a genetic sudden cardiac death screening panel in order to predict risk in patients.
Positive initial results with the Niobe ES system (Stereotaxis) in Europe demonstrate that the average time for completion of mapping and ablation for the atrial fibrillation patients was 69 minutes.
A catheter ablation treatment of a patient with a heart rhythm disorder could be followed via social media, before, during and after the procedure. The initiative is lead by Catharina Hospital in The Netherlands and Philips.
Under the terms of the agreement, Biosense Webster will offer a suite of GE electrophysiology systems, including CardioLab EP recording system, and GE will distribute Biosense Webster's navigation system, the Carto 3 system.
Four interventions to enhance the care and treatment of individuals with atrial fibrillation were presented on 10 January 2011 at a press briefing hosted by The American College of Physicians Foundation's initiative on atrial fibrillation and stroke prevention.
The Sensei X robotic catheter system (Hansel Medical) is a flexible robotic platform that integrates advanced catheter control with 3D visualisation, designed to provide catheter stability with force sensing, instinctive and precise catheter placement.
The premarket application (PMA) submission included data from a 330-patient pivotal IDE clinical study which evaluated the safety and efficacy of the S-ICD system in patients at risk of sudden cardiac death.
In preliminary recommendations published on 9 January, NICE is asking Bayer HealthCare for more information on its product rivaroxaban (Xarelto), for the prevention of stroke and systemic embolism in people with atrial fibrillation.
The anticoagulant dabigatran (Boehringer Ingelheim's Pradaxa) is associated with an increased risk of myocardial infarction or acute coronary syndrome in a broad spectrum of patients when tested against some other medicines, according to a study published online by the Archives of Internal Medicine.
Rhythmview (Topera Medical) is a mapping system that helps identify the electrical source of cardiac arrhythmias. It will be utilised as part of a complex procedure demonstration on 12 January at the 17th Annual Boston Atrial Fibrillation Symposium.
The applications which include AirStrip OB, AirStrip Cardiology and AirStrip Patient Monitoring give clinicians anytime, anywhere access to vital live and historical patient data including waveforms, ECGs and other information, right on their smartphone or tablet.
Researchers from USA have found differences in gene expression that could indicate a predisposition for histiocytoid cardiomyopathy (HC) in infants.
New website is dedicated to updating the physician community on Riata silicone lead information.
The Catheter Guidance Control and Imaging system allows robotic guidance, control and imaging of catheters and other advanced tools used in electrophysiological and other procedures.
Dronedarone (Multaq, Sanofi Aventis) should not be prescribed to patients with atrial fibrillation who cannot or will not be converted into normal sinus rhythm, FDA advises.
Cardiologists from the University of California, Los Angeles (UCLA) have found that surgery to snip nerves related to the sympathetic nervous system on both the left and right sides of the chest, may be helpful in stopping electrical storms when other treatment methods have failed.
Rivaroxaban (Xarelto, Bayer Healthcare) has been approved in the UK for the prevention of non-valvular AF-related stroke and systemic embolism and treatment of deep vein thrombosis (DVT) and prevention of recurrent DVT and pulmonary embolism following an acute DVT in adults.
The survey a joint initiative of the Heart Failure Association and European Heart Rhythm Association of the European Society of Cardiology (ESC) gathered information on more than 2000 patients at 141 centres in 13 European countries.
AtriCure's Synergy Ablation system has been approved for the treatment of patients with persistent and long-standing persistent atrial fibrillation during open-heart concomitant coronary artery bypass grafting and/or valve replacement or repair procedures.
Initial shipments include system upgrades to the new generation Epoch platform from the Niobe II navigation system for customers in the USA and Europe.
A new study in the European Heart Journal indicates that while restrictions on the use of cycloocygenase (COX)-2 inhibitors may have limited the feared cardiovascular consequences of the drugs, the risk of atrial fibrillation may have been overlooked and may warrant further consideration.
Stefan Sack, Munich, Germany speaks to Cardiac Rhythm News about what current data for remote monitoring shows and what studies on remote monitoring are currently investigating.
Lara Dabiri Abkenari, in association with Luc Jordaens at the Erasmus Medical Center in Rotterdam, The Netherlands implanted the 1000th patient with the S-ICD system (Cameron Health).
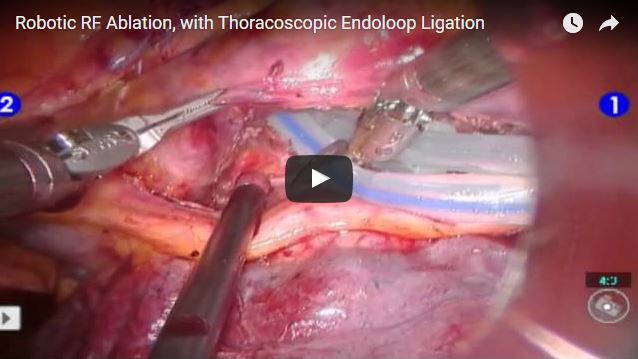
Husam Balkhy, director Center for Minimally Invasive Cardiac and Robotic Surgery, The Wisconsin Heart Hospital, USA, performs a totally endoscopic robotic RF ablation procedure using the Cobra Adhere XL (Estech) with thoracoscopic endoloop ligation of the left atrial appendage.
https://youtu.be/5YMEBORoXXE
The HISTORIC-AF staged hybrid ablation study will enrol persistent and long-standing persistent atrial fibrillation (AF) patients at ten centres in Italy, Germany, the United Kingdom, France, and Poland.
A new study in the European Heart Journal shows that Biotronik's Home Monitoring system is as safe as standard follow-up in patients with pacemakers.
While the panel agreed that the CardioMEMS technology is safe with a vote of 9-1, the majority did not vote positively that the system is effective and that reasonable assurance of the risks associated with the device outweigh the potential clinical benefits of the technology.
Following post-market reports of severe bleeding with dabigatran (Pradaxa, Boehringer Ingelheim), the FDA is to investigate whether the reports show that severe bleeding is more frequent than would be expected given the results of the RE-LY study.
FDA's Circulatory Systems Devices Advisory Panel determined that the overall clinical benefits of Medtronic cardiac resynchronisation therapy with implantable cardioverter defibrillator (CRT-D) devices outweigh the risks in treating certain mildly symptomatic heart failure patients.
"The advantages of speckle tracking echocardiography (STE) over magnetic resonance imaging (MRI) is that it is far quicker to use, cheaper, and can be used by cardiologists at the bedside with portable machines, and repeated serially when ever needed," said Luigi Badano, University of Padua, Italy.
Testing on iCell Cardiomyocytes can help researchers detect potential cardiac safety issues with drug candidates early in the discovery process before in-vivo animal or human testing is conducted.
ALERTS will evaluate the safety and effectiveness of the AngelMed Guardian implantable cardiac monitor and alert system, designed to reduce patient time to the emergency room and improve survival rates from heart attack in high-risk patients.
A trial, simultaneously published online in Circulation and presented at the annual scientific sessions of the American Heart Association (AHA; 12-16 November, Orlando, Florida) has found that minimally invasive surgical ablation is more effective than catheter ablation but is also associated with more procedure-related adverse events
A study presented at the recent 2011 AHA sessions and published online in Circulation has found that the new antiarrhythmic drug celivarone does not prevent implantable cardioverter defibrillator (ICDs) interventions or sudden cardiac death, ending hopes that the drug would be a new weapon in the antiarrhythmic drugs armoury.
At Europe AF, 21-22nd November, London, UK, John Camm (professor of clinical cardiology, St George's University, London, UK) reviewed the available data for vernakalant (Brinavess; MSD, Cardiome Pharma)
A study published in BMJ Open has found that although the number of strokes has fallen in the UK, more needs to be done to reduce the risk of stroke in patients with atrial fibrillation. Additionally, it seems that while the use of anticoagulants does not increase with increasing CHADS2 (congestive heart failure, hypertension, age, diabetes, stroke) score in patients with atrial fibrillation, the use of antiplatelets does increase
MedSolutions, a leading provider of quality-driven medical cost management services, announced the launch of its Implantable Cardioverter Defibrillator (ICD) Surgery Management Program, which uses evidence-based guidelines to ensure the clinical appropriateness of ICD and cardiac resynchronization therapy defibrillator (CRT-D) implantation.MedSolutions, a leading provider of quality-driven medical cost management services, announced the launch of its Implantable Cardioverter Defibrillator (ICD)
Biotronik announced the first implantations of the new Lumax 740 implantable cardiac defibrillators in European countries. According to the company, this is the world's first and only ICDs eligible for use with magnetic resonance imaging.
According to the company, the HeartStart FR3 significantly reduces deployment time by eliminating steps to help responders start delivery of the right therapy-cardiopulmonary or defibrillation- on the patient faster.
The Watchman device (Boston Scientific) is designed for use in patients with atrial fibrillation who are at risk for stroke and are eligible for long-term oral anticoagulation therapy such as warfarin. The device is designed to close the left atrial appendage therefore could be used as an alternative to long-term anticoagulation therapy.
The study aims to demonstrate the safety and efficacy of the CorMatrix extracellular matrix (ECM) for pericadial closure to reduce the incidence of new onset postoperative atrial fibrillation following isolated primary coronary artery bypass graft (CABG) surgery.
Survival curves showed an 80% reduction in total mortality at 90 days for LifeVest patients as compared to non LifeVest patients post percutaneous coronary intervention, with 90 day mortality of 2% for LifeVest patients versus 10% for non-LifeVest patients.
The CRI Amigo system, compatible with certain Boston Scientific and other commercially available catheters, lets physicians remotely maneuver diagnostic and ablation catheters designed to treat common cardiac arrhythmias during electrophysiology procedures.
Pradaxa (dabigatran etexilate) 150mg bid reduced the risk of stroke in atrial fibrillation patients with symptomatic heart failure and non-valvular atrial fibrillation compared to well-controlled warfarin.
Results from the ATLAS ACS 2 TIMI 51 study showed that both 2.5 or 5 mg of rivaroxaban dosed twice daily in addition to standard therapy was superior to standard therapy alone in the primary efficacy endpoint of preventing cardiovascular death, myocardial infarction or stroke in patients with acute coronary syndrome.
In a final draft guidance published on 1 November the UK National Institute for Health and Clinical Excellence (NICE) has recommended dabigatran (Pradaxa, Boehringer Ingelheim), in accordance with its licensed indications, for the prevention of stroke and systemic embolism in people with atrial fibrillation.
A study published in the Annals of Internal Medicine, the largest published experience of MRI in standard, contemporary cardiac rhythm management devices (CRMDs) to date, shows that MRI can be done safely, with appropriate precautions, in patients with selected cardiac devices.
A study, published in Heart, indicates that the combined assessment of left atrial size and the amount of left atrial fibrosis may improve the identification of patients who have a high likelihood of successful ablation.
Biotronik presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), Madrid, Spain, information highlighting the healthcare burden of following-up pacemaker, implantable cardioverter-defibrillator (ICD) and cardiac resynchronisation therapy (CRT) patients in Germany and the UK.
The Food and Drug Administration's approval is largely based on the results from the ROCKET-AF trial, a double-blind global phase III study that showed the safety and efficacy of rivaroxaban (Xarelto, Bayer Healthcare).
The primary objective of the study is to evaluate the percentage of patients implanted with an implantable cardioverter-defibrillator (ICD), featuring the Sorin PARAD discrimination algorithm, that are free from inappropriate ICD shocks over a one-year follow-up.
The majority of the panel does not believe there is reasonable assurance of the safety of Medtronic's ablation system, and that the clinical benefits of the technology do not outweigh the potential risks based on the TTOP-AF clinical trial data.
FDA's Circulatory System Devices Panel recommended AtriCure's Synergy ablation system for the treatment of atrial fibrillation during open-heart concomitant surgical procedures.
Warfarin "swingers"-patients who frequently fall outside of the therapeutic range (international normalised ratio, INR, 2-3) for optimum warfarin control-are a group likely to benefit from being switched from warfarin to a novel oral anticoagulant, according to Ian Menown, Cardiologist, Craigavon Cardiac Centre, Northern Ireland.
Tim Betts, UK, outlined a business case, at the Heart Rhythm congress (2-5 October 2011, Birmingham, UK) to put to commissioners, for using the percutaneous left atrial appendage (LAA) occlusion device (Watchman, Boston Scientific) for stroke prevention in patients with atrial fibrillation.
A study of a Finish population, published online in Heart Rhythm, indicates that cardiomyopathy associated with obesity, alcoholic cardiomyopathy, and fibrotic cardiomyopathy are common causes of nonischaemic sudden cardiac death.
The Early robotic ablation by substrate elimination of ventricular tachycardia study (ERASE-VT), is planned to enrol 200 patients with implantable cardioverter defibrillators (ICDs) at up to eight sites in Europe.
Rainer Moosdorf, Germany, reviews the impact of the STOP VT trial. As reported in the 14th issue of Cardiac Rhythm News, this multicentre study showed that remote magnetic navigation may provide additional benefits to catheter ablation for the treatment of scar-related ventricular tachycardia.
Despite significant improvements in stroke prevention over the past decade, and a fall in incidence and deaths, UK doctors are still undertreating one of the major risk factors-atrial fibrillation-reveals research published in BMJ Open.
At a median follow-up time of 36 months, patients with an abnormal Microvolt T-Wave Alternans (MTWA) test were 4.4 times more likely to experience a life-threatening arrhythmia or sudden cardiac death than those with a normal test and were almost eight times more likely to die of cardiac causes, according to results from the PREVENT-SCD trial.
Further research presented during the scientific sessions showed that the CardioFocus' balloon system achieved 99% acute pulmonary vein isolation irrespective of vein anatomy.
SonR is the first and only system to provide weekly automatic optimisation during patient's real life activities as an alternative to in-clinic manual echocardiography-based device optimisation for improved cardiac resyncronisation therapy response.
The new additions provide physicians easier access to important alerts and improve efficiency of data transmission to electronic health records.
The TTOP-AF trial results showed a 90% reduction in atrial fibrillation or atrial flutter burden in 55.8% of ablation patients; however the results did not meet the pre"defined performance safety goal of 16%.
The endoscopic ablation system which is used for pulmonary vein isolation in the treatment of atrial fibrillation, will be highlighted in a "live case" session performed by Sakis Themistoclakis and Andrea Natale
Roberto M Lang, professor of Medicine, University of Chicago Medical Center, Chicago, USA, is a pioneer in the development of 3D echocardiography. Lang, who is past president of the American Society of Echocardiography, spoke to Cardiovascular News about the...
Despite influencing the epidemiology, pathology, and clinical presentation of cardiac arrhythmias, gender does not appear to affect periprocedural complications and outcomes of catheter ablation, according to a study presented by Sonia Ammar, Munich, Germany at EHRA-Europace in Madrid, Spain.
There is ongoing debate as to whether or not young athletes should undergo preparticipation screening, using an electrocardiogram (ECG), to reduce the risk of sudden cardiac death. A new study indicates that preparticipation ECGs are difficult to interpret and as a result, the wrong guidance may be given.
Will Nicolson, University of Leicester, UK, comments on the findings from the two new studies on electrocardiograms for preparticipation screening in young athletes published in the Journal of Pediatrics and Circulation. He said that according to the results "it is possible that preparticipation ECG screening would cause more long-term deaths through inactivity than it saved through appropriate diagnosis in the short term."
Andrea Natale, co-president of Venice Arrhythmias (9-12 October 2011), is one of the pioneers in ablation of atrial fibrillation, he spoke to Cardiac Rhythm News about this as one of his proudest moments, he also told who inspired him into electrophysiology and what is expected for Venice Arrythmias 2011.
The Quick View report is designed to improve the speed at which clinicians can find and review patient device (pacemaker or implantable cardioverter-defribrillator) data by providing a very intuitive, user-friendly presentation of the information.
One Heart Magazine, the voice of the Global Cardiovascular Alliance, will highlight corporations, nonprofit organisations, individuals, and strategic alliances that are working to further the cause of this charitable foundation dedicated to provide cardiovascular implantable devices and treatment to people in need around the world.
According to the Agency, the anti-arrhythmic medicine should only be prescribed for maintaining heart rhythm in patients with paroxysmal or persistent atrial fibrillation for the maintenance of sinus rhythm after successful cardioversion.
Remanic provides an optimised user interface to increase patient's follow-up efficiency as well as a retractable and adjustable touchscreen for optimised viewing and handling.
Keltjens has previously served as chief executive officer of AngioDynamics and CryoCath Technologies (now Medtronic CryoCath) and held a number of senior leadership positions at Cordis.
A new US study shows that infections associated with pacemakers and defibrillators led to 4.8 to 7.7-fold increases in admission mortality, 1.6 to 2.1-fold increases long term mortality, 2.5 to 4.0-fold increases in hospital length of stay, and 1.4 to 1.8-fold increases in cost compared to pacemaker and defibrillator implantations without infection.
The Promote Quadra CRT-D quadripolar pacing system offers physicians the ability to address pacing complications without the need to surgically reposition a lead.
More than 1,000 cardiac arrest deaths over 15 years are connected to the failure of automated external defibrillators (AEDs), according to a study published in Annals of Emergency Medicine.
The US Food and Drug Administration's Cardiovascular and Renal Drugs Advisory Committee recommended approval of rivaroxaban (Xarelto, Bayer) for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation.
The first patient has been enrolled in the Analyze ST clinical study to evaluate the effectiveness of an ST segment monitoring feature in St Jude's Fortify ST implantable cardioverter defibrillator (ICD) to detect acute coronary events.
New data from the ARISTOTLE study showed that the rate of haemorrhagic stroke in the apixaban group was 49% lower and the rate of ischaemic stroke was 8% lower than in the warfarin group. Results were presented by Christopher Granger, USA, at the European Society of Cardiology (ESC) Congress (27-31 August 2011, Paris, France).
The ECOST and the EVATEL studies presented at the ESC Congress, Paris, France, show that remote monitoring (of cardiac rhythm management devices) reduces the number of inappropriate shocks compared with standard follow-up.
"The main advantage of the Niobe Remote Magnetic Navigation System (Stereotaxis), is the precise mapping and identification of the critical diseased portions of the heart that allows physicians to perform ablation in a more effective way," said Petr Neuzil, lead investigator of the STOP-VT study presented at the ESC Congress in Paris.
The two-year results are consistent with the findings of the original PACE study: that biventricular pacing provides protection against the adverse effects of right ventricular apical pacing on systolic function and remodelling.
The study has indicated that enhanced atrioventricular search hysteresis algorithm reduces unnecessary right ventricular pacing which correlates with less risk of heart failure hospitalisation, mortality and atrial fibrillation.
Selectra's unique handle design has an integrated side port, allowing for maximum flexibility and one-handed manipulation, which results in ultimate convenience during even the most challenging implantations.
ECOST is the first trial to show a 52% reduction of the number of patients with inappropriate shocks and a 72% reduction in the risk of hospitalisations related to inappropriate shocks.
Eliquis (Bristol-Myers Squibb and Pfizer), compared to standard of care warfarin, significantly reduced risk of stroke or systemic embolism by 21%, risk of major bleeding by 31% and mortality by 11%.
Results from the Home-CARE (Home monitoring in cardiac resynchronisation therapy) clinical study showed that combining several physiological parameters into a single algorithm may help to improve the early detection of cardiovascular events.
"The findings of this analysis demonstrate that rivaroxaban has the ability to prevent stroke in patients with moderate renal insufficiency, without elevating the risk of critical bleeding events such as intra-cranial haemorrhage," said Keith Fox at the European Society of Cardiology Congress in Paris.
Biotronik announced the list of its sponsored clinical studies on remote monitoring in ICD and CRT-D patients to be presented at the European Society of Cardiology (ESC) Congress to be held in Paris, France, from 27-31 August 2011.
HeartLight EAS (CardioFocus) is used for the treatment of atrial fibrillation. It features an illuminating endoscope to provide physicians with direct visualisation within a beating heart, in real time and without radiation.
The EPi-Sense (nContact) features first-of-its kind epicardial sensing electrodes designed to allow both electrophysiologists and cardiothoracic surgeons to map and navigate the epicardium and locate areas for linear epicardial ablation.
In response to the cardiac industry trend of shifting patient care from health-care settings to home, Centauri (IntriCon) gives physicians the flexibility to identify and monitor asymptomatic patient cardiac arrhythmias remotely.
The recommendations made in the guidance are intended to improve the predictability, consistency and transparency of the premarket review process for applicable devices, and should help manufacturers navigate the approval process more easily.
A novel nitinol guidewire (SafeSept, Pressure Products) may be an alternative to transoesophageal or intracardiac echocardiogram for transseptal puncture for left atrium catheter ablation, study shows.
The recommended dose, Pradaxa 150mg twice daily, is licensed in the United Kingdom for the prevention of stroke and systemic embolism in eligible adult patients with atrial fibrillation.
A large, multi-year, biracial study is first to show more than a two-fold increase of incident atrial fibrillation in current smokers compared to those who have never smoked.
A new technique that stimulates heart muscle cells with low-energy light raises the possibility of future light-controlled, long-lasting pacemakers and defibrillators, the technique might also be used to test new drugs for possible cardiac side effects, study shows.
The EAST clinical trial is the largest pan-European study to evaluate whether an early and comprehensive rhythm control treatment of patients with early onset atrial fibrillation produces better patient outcomes than usual care alone.
ROCKET-AF co-chair Keith Fox, from the Centre for Cardiovascular Science at the University of Edinburgh, UK, talks to Cardiac Rhythm News about the study and its implications.
Arrhythmia Alliance (A-A) and sister charity, Syncope Trust And Reflex Anoxic Seizures (STARS) surveys' results will be used to help inform, educate and raise awareness of heart rhythm disorders and syncope globally.
Rivaroxaban (Xarelto, Bayer) successfully met the primary efficacy endpoint of non-inferiority in the prevention of stroke and non-central nervous system embolism in non-valvular atrial fibrillation, with superior efficacy demonstrated compared to warfarin whilst on active treatment.
Following the early termination of the PALLAS trial, the Guidelines Department of the European Society of Cardiology (ESC) has issued a new statement with regards to the use of dronedarone in atrial fibrillation patients, in accordance with advice given by the European Medicines Agency and the US Food and Drug Administration.
Medtronic will conduct a prospective, randomised, controlled, non-blinded, multicentre clinical trial to confirm the safety and effectiveness of its second generation pacing system, in the clinical magnetic resonance imaging setting under specified conditions.
"FDA believes that the 510(k) process should not be eliminated but we are open to additional proposals and approaches for continued improvement of our device review programmes," said Jeffrey Shuren, director of the FDA's Center for Devices and Radiological Health.
Researchers from the University of Pennsylvania found that previous myocardial infarction, heart failure, atrial fibrillation, physical inactivity, diabetes, and reduced kidney function are all closely associated with sudden cardiac death.
The 510(k) process lacks the legal basis to be a reliable premarket screen of the safety and effectiveness of moderate-risk Class II devices and cannot be transformed into one, according to the US Institute of Medicine of the National Academies of Science.
The development of this novel catheter, specially designed to be used with magnetic resonance imaging, could represent a significant new opportunity for improved therapy of complex cardiac arrhythmias.
Thomas Moyer, Mayo Clinic, Department of Laboratory Medicine and Pathology, describes the four basic categories of patients as identified through a sensitive genotype test, and how the doses of the blood-thinner warfarin would typically be adjusted to reflect differences...
Spontaneous circulation was restored three times more commonly with adrenaline (23.5%) than with saline placebo (8.4%) after cardiac arrest treatment, according to a study to be published in the journal Resuscitation.
Carlo Pappone, The San Raffaele Hospital, Milan, Italy, performs atrial fibrillation ablation with Stereotaxis remote magnetic navigation system and CARTO RMT non fluoroscopic mapping system.
https://youtu.be/p1BZWQW3Zmc
Specific kinds of labeling changes, changes to the technology used in the device, changes in performance specifications, manufacturing changes, and changes in the materials used in the manufacture of the device are part of the clarifications included in the FDA's draft guidance.
The number of deaths in the dronedarone (Multaq, Sanofi Aventis) arm of the recently halted PALLAS study was double the number of deaths in the control arm, according to figures released by the US Food and Drug Administration (FDA).
New data from the MADIT-CRT study shows that cardiac resynchronisation therapy with a defibrillator (CRT-D) significantly improves both left ventricular dyssynchrony and contractile function in patients with class I or II heart failure.
The new method, developed by Cornell University scientists, terminates the turbulent electrical activity within the heart. The weak electrical signals create "virtual electrodes" that stimulate heterogeneities within the heart and, particularly, the blood vessels.
The SMART-AF investigational device exemption study is designed to demonstrate the safety and effectiveness of ThermoCool SmartTouch and FDA approved NaviStar ThermoCool catheter family, in the treatment of symptomatic, drug-refractory, paroxysmal atrial fibrillation.
The new software is designed to give electrophysiologists access to contact-force information in a fluoroscopically enabled, three-dimensional anatomic heart model with X-ray based catheter tracking system.
This follows Sanofi's announcement on 7 July 2011 of its discontinuation of the PALLAS study, because of the occurrence of severe cardiovascular events in some patients taking Multaq.
The decision follows recommendations from the PALLAS trial's Operations Committee and the Data Monitoring Committee (DMC) which observed a significant increase in cardiovascular events in the dronedarone arm.
The agreement will integrate the contact-force data provided by Endosense into GE's CardioLab electrophysiology recording and Innova imaging platforms.
Despite recent advances in the treatment of heart rhythm disturbances, mortality and morbidity rates associated with AF remain unacceptably high, according to a joint report by the German Competence Network on Atrial Fibrillation (AFNET) and the European Heart Rhythm Association (EHRA).
The popularity of remote monitoring systems for implantable cardiac rhythm management devices is slowly increasing, according to Vidal Essebag, McGill University, Montreal, Canada.
John Camm, St Georges University of London, United Kingdom, overviewed the incidence of sudden cardiac death in Europe and USA at the European Heart Rhythm Association (EHRA)-Europace Congress in Madrid, Spain (26-29 June 2011)
The take-home message from a session on the electrophysiological aspects in stem cell therapy at the recent EHRA-Europace Congress (Madrid, Spain) is that more research is needed to resolve the issue of the risk of arrhythmias with the use of stem cells in myocardial repair.
In this study of patients with atrial fibrillation and at least one additional risk factor for stroke, Eliquis (Bristol-Myers and Pfizer) met the primary efficacy objective of non-inferiority to warfarin on the combined outcome of stroke.
Under the agreement, EHRA-Europace and Cardiostim share a wish to avoid fragmentation and create a common vision and objective - to promote the field of electrophysiology.
Medtronic announced the launch of Medtronic's first mobile application for use with implantable cardiac devices. With access to alert transmissions via the CareLink mobile application, health care providers can now manage their patients anytime and anywhere.
The reduced footprint of the Fortify ST implantable cardioverter defibrillator (ICD) and Unify cardiac resynchronisation therapy defibrillator (CRT-D) makes them the smallest available in the industry.
Hein Heidbuchel, director of Electrophysiology at the University Hospital Leuven, spoke to Cardiac Rhythm News about his career, influences and proudest moments. He also described the innovations that shaped his career, new technologies and EP education.
As the 2011-2012 president takes office, Bruce L Wilkoff speaks about his goals for the Heart Rhythm Society.
In patients affected by permanent atrial fibrillation (AF), cardiac resynchronisation therapy (CRT) proved superior to conventional right ventricle apical pacing in reducing the clinical manifestations of heart failure, a new study presented at Heart Rhythm 2011 has shown.
The subcutaneous implantable cardiac defibrillator (S-ICD) system is a viable alternative to conventional ICD systems for selected patients, results from a Dutch experience have shown.
Meet the new Heart Rhythm Society's president 2011-2012: Bruce Wilkoff, director of Cardiac Pacing and Tachyarrhythmia Devices in the Department of Cardiovascular Medicine at the Cleveland Clinic.
The S-ICD system (Cameron) is the only implantable defibrillator that does not require electrical wires in the heart. This system is implanted using a completely subcutaneous procedure that leaves the heart and blood vessels untouched and intact.
VantageView system includes a 56 inch high-definition monitor that can display up to eight video images simultaneously.
Baxter International launched Nexterone (amiodarone HCl) premixed injection, the first and only ready-to-use premixed intravenous bag version of the antiarrhythmic agent amiodarone in the United States.
The C-Pulse system is an investigational device designed to relieve the symptoms of heart failure, such as shortness of breath and reduced mobility, through the use of counter-pulsation technology.
Survival at exercise facilities was 50%, compared with 36% observed for sudden cardiac arrest occurring in non-exercise public indoor locations. The study was presented at Heart Rhythm 2011, the Heart Rhythm Society's 32nd Annual Scientific Sessions
Energen and Punctua cardiac resynchronisation therapy defibrillators (CRT-Ds) and implantable cardioverter defibrillators (ICDs) are the world's smallest and thinnest high-energy devices to treat heart failure and sudden cardiac death and offer excellent longevity.
The incidence of silent cerebral lesions after pulmonary vein isolation is different depending on the technology used, a new study has shown. Atrial fibrillation patients treated with multi-electrode PVAC showed higher incidence of silent cerebral ischaemic events compared to irrigated radiofrequency and cryoballoon ablation.
In the first randomised clinical trial to report the benefits of using echocardiography to guide placement of pacemaker leads, researchers found that a patient-tailored approach using software to analyse left ventricle function and guide placement of lead wires can significantly boost clinical benefits from pacemakers.
Alon Barsheshet, University of Rochester Medical Center, Rochester, USA, spoke to Cardiac Rhythm News on how right ventricular pacing is associated with an increased risk of heart failure.
Fiorenzo Gaita, Università degli Studi di Torino, Turin, Italy, spoke to Cardiac Rhythm news about the real impact of fluoroscopy, intracardiac echocardiography and virtual 3D electro-anatomic mapping in atrial fibrillation ablation.
Achieve is an intra-cardiac electrophysiology diagnostic catheter that can be used to assess pulmonary vein isolation when treating paroxysmal atrial fibrillation (PAF). The first patient procedure in the United States using this catheter was performed by Robert Kowal, electrophysiologist at the Baylor Heart & Vascular Hospital in Dallas, USA.
Sorin's latest devices include SafeR and Parad+ algorithms that automatically adjust to each individual patient to minimise right ventricular pacing and inappropriate shocks.
Attain Ability Plus enables physicians to reach and maintain the target vein, providing stability in medium-to-large venous anatomies, while Attain Ability Straight allows physicians to maneuver through small cardiac veins.
Douglas Packer spoke to Cardiac Rhythm News about his career, family, his presidency of the Heart Rhythm Society, new technologies and the CABANA trial.
A multicentre, prospective study confirms lack of predictive accuracy of ventricular tachycardia/ventricular fibrillation (VT/VF) inducibility and identifies novel risk indicators for arrhythmic events in Brugada patients. Results of the PRELUDE registry were presented at Hearth Rhythm 2011, San Francisco, USA.
Results from the CONFIRM trial showed that a targeted, single procedure ablation at identified sources increased the long-term freedom from atrial fibrillation by 70% over the existing standard of care.
The distance at which headphones cause magnetic interference with active implanted cardiac medical devices (AICMDs), such as implantable cardiac pacemakers (ICPs) and cardioverter defibrillators (ICDs), is less than previously reported, according to a research presented at Heart Rhythm 2011, San Francisco, USA.
Boston Scientific's latest radiofrequency ablation catheter is designed to treat a variety of arrhythmias and integrates Total Tip Cooling design which is intended to consistently cool the entire tip electrode during radiofrequency energy delivery.
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) has recommended approval of dabigatran etexilate (Boehringer Ingelheim's Pradaxa) in the member states of the European Union for the prevention of stroke and systemic embolism in adult patients with nonvalvular atrial fibrillation with one or more risk factors.
Compliance with the implantable cardioverter defibrillator process measure in eligible patients was associated with 38% lower odds of mortality over two years, and cardiac resynchronisation therapy process measure compliance was associated with 36% lower odds of mortality.
The technology, which can be used with new Unify cardiac resynchronisation therapy defibrillators and Fortify implantable cardioverter defibrillators, is designed to reduce inappropriate and unnecessary shocks for patients with these devices.
Greater health care expenditure supported by robust economic growth will lead to nearly 13% annual growth in the market for pacemakers, implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy devices in China, according to Millennium Research Group.
REVOLUTION is a prospective, multicentre, non-randomised clinical study for the evaluation of a multi-electrode pulmonary vein isolation system used to treat paroxysmal atrial fibrillation.
Medtronic's CRT-P systems are the first to offer exclusive Optivol fluid status monitoring with complete remote follow-up for proactive heart failure management.
Jared Bunch, cardiologist and electrophysiologist, Intermountain Medical Centre, Utah, USA, explained to Cardiac Rhythm News how atrial fibrillation is independently linked to memory impairment, cognitive decline, and general dementia.
Even moderate alcohol consumption can lead to the development of atrial fibrillation, according to researchers from the University of Tsukuba, Institute of Clinical Medicine, Mito, Japan.
The new tool has all necessary medical devices printed on a standard balloon catheter: a device for eliminating damaged tissue using heat, temperature and pressure sensors, a light-emitting diode (LED) and an electrocardiogram sensor.
The Tempasure system is the world's first radiofrequency cardiac ablation catheter to offer both saline irrigation and temperature-sensing technology for the treatment of atrial arrhythmias.
J Alberto Lopez, a physician affiliated with Texas Heart Institute at St. Luke's Episcopal Hospital, is the first in Houston and among the first in the nation to treat patients with paroxysmal atrial fibrillation with cryoablation technology.
Todd Cohen, director of Electrophysiology and Pacemaker/Arrhythmia Center, Winthrop-University Hospital, USA, completed the procedure and was also the lead investigator in the clinical trials that resulted in FDA approval for the Revo MRI Sure Scan (Medtronic) in February 2011.
The first catheter ablation procedure was performed at the end of February by Vivek Y. Reddy, director of the Cardiac Arrhythmia Service at Mount Sinai Heart and colleagues.
Female patients had a better result from cardiac resynchronisation therapy with defibrillator than male patients with a significant 69% reduction in death or heart failure, according to results published in the Journal of American College of Cardiology.
Despite major advances during the past two decades in the management of patients with acute myocardial infarction, sudden cardiac death remains an important contributor to overall mortality, according to Reginald Liew, National Heart Centre, Singapore.
The Medtronic's mapping catheter is an intra-cardiac electrophysiology recording catheter that can be used to assess pulmonary vein isolation when treating paroxysmal atrial fibrillation.
Administering GRNCM1 (Gerom) by injection into the heart resulted in greater resistance to induced arrhythmias, halted adverse cardiac remodeling and preserved mechanical function compared to controls.
Renamic is used for initial programming, reprogramming and testing of implantable pacemakers, implantable cardioverter-defibrillators (ICDs) and cardiac resynchronisation therapy (CRT) devices after implantation.
The goal of the HeartRescue Project is to improve out-of-hospital cardiac arrest survival rates by at least 50% in five years, within five pilot states in USA.
The new transseptal module offers a complete training curriculum for electrophysiologists on the Angio Mentor, allowing physicians a safe environment in which to train until proficient in the essential tasks they are required to perform.
These markets could reach over €4.4 billion by 2017, according to a report published by iData Research.
Peter Schwartz tells Cardiac Rhythm News about loving his research, being best man at a patient's wedding and having an article about golf published in a medical journal.
Proceeds support initiation of US pivotal trial and expansion of European commercialisation of the CardioFocus' Endoscopic Ablation System.
This trial investigates whether implantable cardioverter defibrillator (ICD) implantation without an intraoperative defibrillation test is noninferior to ICD implantation with intraoperative defibrillation testing.
EFFICAS I is a 45-patient, single-arm, prospective, multicentre European clinical trial designed to demonstrate the correlation between contact forces applied during pulmonary vein isolation and atrial fibrillation treatment efficacy at three months.
The newly approved drug dabigatran is an alternative to warfarin to help prevent dangerous blood clots in patients with atrial fibrillation (AF), according to updated guidelines from the American College of Cardiology, American Heart Association and the Heart Rhythm Society.
The campaign aims to promote education around the risks associated with AF as well as highlight the importance of early diagnosis and comprehensive management to maximise patient outcomes.
Ali Al-Sayegh, cardiologist at the Chest Diseases Hospital, Kuwait, explains how cardiac resynchronization therapy has better outcomes using quadripolar LV leads.
The Arctic Front Cryoballoon from Medtronic is safe with very low incidence of pulmonary vein stenosis and no cryo-related left atrial perforation and low thrombogenic risk. Results were presented by Wyn Davies, London, UK, at the annual Europe AF meeting in London, UK.
Bristol-Myers Squibb and Pfizer announced the full results of the AVERROES study of apixaban in The New England Journal of Medicine.
None of the 211 patients who underwent a magnetic resonance imaging exam during the clinical trial of Revo MRI SureScan pacing system experienced an MRI-related complication, the FDA stated.
A total of 20,240 patients with non-valvular atrial fibrillation (AF) were assessed for eligibility in the RE-LY trial. Gregory Lip, University of Birmingham, UK, presented the results of the RE-LY sub-studies at the annual Europe AF meeting in London.
In atrial fibrillation (AF) patients unsuitable for a vitamin K antagonist, apixaban reduces stroke by more than 50% compared to acetylsalicylic acid without a significant increase in major bleeding.
Reliaty is used to ensure adequate lead placement of implantable pacemakers, Implantable Cardioverter-Defibrillators (ICDs) and Cardiac Resynchronisation Therapy (CRT).
The main goal of the shared development project is to achieve an improved visualisation of catheter-tip-to-tissue contact force within a three-dimensional anatomic heart model during catheter ablation procedures.
The Atritech Watchman left atrial appendage closure device is an alternative to warfarin for patients experiencing atrial fibrillation who are at high risk for stroke.
The new drug's label warning is due to two cases of acute liver failure leading to liver transplant in patients treated with this heart medication.
The CE-marked Thermocool Smarttouch catheter provides an important new parameter for the mapping and ablation of complex cardiac arrhythmias, such as atrial fibrillation.
TOCCASTAR will explore a new catheter ablation treatment option for patients who have symptomatic paroxysmal atrial fibrillation and who are resistant or intolerant to at least one Class I-IV anti-arrhythmic drug.
Vdrive combines magnetic navigation with the Odyssey information management system to bring precise control during catheter-based electrophysiology procedures.
Clinical studies on rivaroxaban have shown no requirement for routine laboratory coagulation monitoring, and limited risk for food and drug interactions.
Panos Vardas from Greece, president of the European Heart Rhythm Association (EHRA) and president-elect of the European Society of Cardiology (ESC) spoke to Cardiac Rythm News about his passion for Cardiology, his biggest achievements and plans to lead the ESC.
Five-year follow-up results from a study of catheter ablation for atrial fibrillation shows long-term success increases when outcomes are measured after the last ablation procedure.
Study published in the JAMA also showed that these patients had a significantly higher risk of in-hospital death than those who met criteria for receiving an ICD.
The Arctic Front Cardiac CryoAblation Catheter system is the first and only Cryoballoon in the United States indicated for the treatment of drug refractory paroxysmal atrial fibrillation.
"This approach provides a single ablative means of eliminating problematic atrial fibrillation in patients who failed drug therapy," said Douglas Packer, cardiologist at Mayo Clinic.
"The new physician specialty code is a major achievement for the Heart Rhythm Society and the field of electrophysiology," said Douglas L Packer, president of the Heart Rhythm Society.
Prospective, multicentre, randomised trial will enrol more than 400 patients at up to 15 cardiac surgery sites in the USA.
Advanced suite of EnSite Velocity System product offerings streamline workflow and provide physicians with improved efficiency.
"CRT not only helps keep less sick heart failure patients out of the hospital more, but it is also a more cost-effective treatment approach than many other therapeutic options currently available," said Cecilia Linde, Stockholm, Sweden.
The analysis also showed that heart failure patients who transmitted weight and blood pressure data via the system experienced an additional 10% reduction in the risk of death.
The CRYSTAL AF trial will enrol approximately 450 people who have been diagnosed with a cryptogenic stroke across 55 centres.
More than 21,000 patients were enrolled in the largest clinical study and were randomised to receive the factor Xa inhibitor or warfarin.
Patients who were treated with catheter ablation reported fewer symptoms and improved quality of life than patients treated with anti-arrhythmia drugs at one year.
Study published in Circulation showed that, after one year, 92% of ICD recipients and 88% of CRT-D patients were alive. Survival rates after five years were 68% for ICD and 54% for CRT-D.
AF AWARE campaign calls for urgent action to improve patients' health and reduce costs to national health systems.
New 24-hour, web-based service helps cardiologists leverage cutting-edge technology in implanted cardiac devices.
Medtronic's PICTURE registry results show that device led to diagnosis and treatment for 78% of patients who experience a recurrent syncopal event.
Incepta, Energen and Punctua CRT-Ds and ICDs designed to advance patient care with options to expand appropriate therapy, optimise ventricular pacing and manage heart failure.
Study shows that cardiac resynchronisation therapy is as effective as two other methods that regulate the time interval between the squeezing of the heart's upper and lower chambers.
Pacemaker patients who have device-detected arrhythmias are approximately 2.5 times more likely to have a stroke than patients who do not have device-detected arrhythmias.
The new drug does not increase bleeding risk among atrial fibrillation patients, according to results of the ROCKET AF trial presented at the American Heart Association's Scientific Sessions.
The FDA approved on 19 October 2010 dabigatran etexilate (Pradaxa, Boehringer Ingelheim) for the prevention of stroke and blood clots in patients with atrial fibrillation.
The BIOTRONIK EchoCRT clinical trial aims to evaluate the impact of cardiac resynchronisation therapy in patients with advance heart failure.
Dabigatran etexilate may offer patients and doctors the first new treatment option for stroke prevention in atrial fibrillation in more than 50 years.
A new indication for three cardiac resynchronisation therapy defibrillators can be used for patients with an abnormality known as left bundle branch block.
The three devices from Boston Scientific are intended to treat patients with left bundle branch block who have either mild heart failure or heart failure with no apparent symptoms.
System supports proactive patient care for patients at risk for worsening heart failure.
Intravenous formulation of vernakalant can offer physicians, patients and hospitals an important therapy option to use for the rapid treatment of recent-onset atrial fibrillation.
Technology appraisal recommends the use of dronedarone in treating Atrial Fibrillation under specific conditions.
Agreement is part of deal to promote new products utilising new complementary technologies tackling heart rhythm disorders.
Cameron Health's S-ICD device promises to reduce the delivery of "unnecessary shocks".
Study shows molecular imaging can play an important role in diagnosing risk in cardiovascular patients, according to researchers in Japan.
Study has been designed to evaluate multiple physiologic sensors in cardiac resynchronisation therapy defibrillators.
Over ten patients enrolled in first-ever multicentre randomised trial comparing atrial fibrillation ablation devices.
Report suggests drug should only be considered as treatment option for people who have additional cardiovascular risks.
Laser Sheath to become company's first-ever Japanese export.
Live demo highlights the benefits of integrated technology.
Reocor offers extended battery service times of up to 25 days.
The company release a new cardiac rhythm management connector system designed to simplify implant procedure.
The Promote Quadra CRT-D should be considered "a new standard of care" says Wolfgang Kranig.
Lumax DX system pioneers the use of 'Smart Detection' in monitoring atrial activity.
Ryszard Piotrowicz, head of Department of Cardiac Rehabilitaion and Noninvasive Electrocardiology, National Institute of Cardiology, Warsaw, explains the advances in the provision of cardiac rehabilitation to heart failure patients.
The US$2.2 million grant will support the design and commercialisation of SPS's revolutionary steerable cardiac mapping and ablation catheter technology.
Richard L Page hands over to Packer at HRS ceremony.
Josep Brugada speaks to Cardiac Rhythm News about his career, his proudest moments and his association with Barcelona FC.
Results show the TactiCath to be an effective ablation catheter.
Gust H Bardy believes S-ICDs to prove a "viable alternative" to the transvenous system.
Lessons in programming to be learnt from findings, claims Bruce Wilkoff.
Riccardo Cappato, Centre of Clinical Arrhythmia and Electrophysiology, Policlinico San Donato, Milan, makes his case following the second global survey on catheter ablation.
Dot Medical hails the Wearable Defibrillator as one of "technologically exceptional".
FDA approval given for the Paradym CRT Model 8750.
CEO praises technological advances in the module's development.
The Fortify and Unify devices get CE Mark; expected to be universally available by the end of the year.
New USB adaptor allows patients using Merlin@home transmitter to transfer data to their physician over mobile phone networks.
Bruce L Wilkoff, The Cleveland Clinic, Cleveland, USA, has been involved in the development of several cardiac devices. He tells Cardiac Rhythm News which of them he considers the most important.
NICE chief executive finds dronedarone "less effective" compared to existing AF treatments.
Professor Douglas Packer presents the results of the pilot study at the American College of Cardiology Scientific Sessions.
Professor Douglas Packer presents the results of two clinical trials conducted using the cryoablation technique.
Physio-Control admits there may be a battery problem with the Lifepak 20/20e defibrillator/monitor.
New option in second-generation pacing system available in select European geographies features exclusive technology and allows access to critical diagnostic tool.
The sub-analysis demonstrated that both men and women experienced benefit from CRT but women experienced a 70% reduction in heart failure events compared to a 35% reduction for men.
The second generation product includes an elegantly enhanced catheter along with a feature-rich supporting system called the TactiSysTM.
Boston Scientific has received FDA clearance for the two validated manufacturing changes affecting all of its CRT-Ds and ICDs.
Biosense Webster announced on 9 April 2010 that it has obtained its first Ethics Committee approval for the study in patients with paroxysmal atrial fibrillation.
Medical device manufacturer will pay criminal penalty of more than US$296 million for violations of the US Federal Food, Drug and Cosmetic Act.
The NICE appraisal committee's preliminary recommendation is to endorse Multaq as a first choice therapeutic option after beta-blockers for non-permanent AF patients.
If approved, Revo MRI has the potential to be the first FDA-approved pacing system designed for use in the MRI setting.
The panel unanimous decision extends resynchronisation therapy for asymptomatic and mild heart failure patients based on landmark MADIT-CRT trial.
Advisa DR MRI SureScan, the most advanced pacing system from Medtronic that combines MRI compatibility with exclusive pacing technology, is now available in Europe.
Safety and effectiveness trial evaluated the Medtronic Arctic Front Cardiac CryoAblation Catheter System for the treatment of paroxysmal atrial fibrillation.
Study published in HeartRhythm Journal shows female patients undergo catheter ablation less often and experience more complications than males
An overview of clinical trials on AF ablation devices seeking FDA approval was presented by Hugh Calkins, Johns Hopkins Hospital, Baltimore, USA, at the Boston AF Symposium.
Results of the ACTIVE A and RE-LY trials were presented by Stuart Connolly, McMaster University, Hamilton, Canada, at the Boston AF Symposium.
New observations from the Second Worldwide Survey on AF Ablation showed that more procedures are being offered every year.
New study will revisit patients from the groundbreaking SCD-HeFT trial to collect long-term data regarding the efficacy of ICDs in heart failure patients.
Under the terms of the agreement, Biotronik will be the exclusive distributor of the TactiCath in all major markets outside of the United States, Japan and Asia.
The FDA also approved all magnetic catheters for use with newly increased field strength for magnetic navigation.
"The evidence provided to the independent appraisal committee indicates that dronedarone is less effective and costs considerably more than existing treatments for controlling AF," the agency wrote.
Diagnostic device for complete wireless remote patient monitoring is compatible with the Biotronik Home Monitoring.
Enhanced visibility and performance features help physicians dilate scar tissue surrounding problematic cardiac leads requiring removal.
Multaq, developed by Sanofi-aventis, is the first new anti-arrhythmic drug to be approved in Europe in the last 10 years.
Cognis cardiac resynchronisation therapy defibrillator and Teligen implantable cardioverter defibrillator are the world's smallest and thinnest high-energy devices.
Charles Love, The Ohio State University, analyses updates and highlights on issues related to lead management and extraction.
Fred Morady spoke to Cardiac Rhythm News about his influences, areas of research, and activities outside medicine: building furniture at home and downsizing to a Cessna 182.
Patients receiving dronedarone for atrial fibrillation on top of adequate antithrombotic medication presented a significantly lower incidence of stroke, according to Stefan Hohnloser.
We have many drugs available to us that could eventually come to clinical trials, but there are very few studies ongoing at the moment, John Camm said at the Venice Arrhythmias symposium.
The clinical trial, sponsored by Boston Scientific, was designed to determine which programming strategy best minimises the occurrence of unnecessary therapy in ICD and CRT-D patients.
Hansen Medical showcased the small, flexible irrigated ablation catheter for the first time. CE mark is expected in the first half of 2010.
Isolation of the pulmonary vein antrum and the superior vena cava using intracardiac echocardiography and circular mapping can achieve encouraging cure rates.
Cardiologist, professor of Medicine at the University of Rochester Medical Center, USA, led studies that have changed medical guidelines.
The Current Accel ICD, AnalyST Accel ICD, Promote Accel CRT-D, Durata high-voltage cardiac lead, and the SJM Confirm implantable cardiac monitor are now approved in the country.
The European Medicines Agency has adopted a positive opinion recommending the grant of a marketing authorisation for Multaq (dronedarone, 400mg tablets).
Panos E Vardas, president of the European Heart Rhythm Association, analyses the trend on expanding the existing indications and usage of ablation.
Asymptomatic or mildly symptomatic cardiac patients randomised to an implanted CRT-D have a 34% lower risk of heart failure or death than those receiving a standard ICD.
The Lifepak 15 provides the widest range of energy dosing up to 360 joules, and the broadest selection of monitoring options available.
Physicians at The Mount Sinai Medical Center in New York became the first in the US to ablate atrial fibrillation using the "Endoscopic Ablation System" manufactured by CardioFocus.
AF Stat Call to Action for Atrial Fibrillation report cites limited understanding, disconnected dialogue and uncoordinated care as key barriers to improved outcomes.
Niraj Varma and Thomas Vogtmann both led studies on the Biotronik Home Monitoring System. They spoke to Cardiac Rhythm News at the ESC 2009 Congress in Barcelona, Spain.
Data from the studies TRUST; REFORM and ISMOS have demonstrated that the Biotronik Home Monitoring is reliable and effective in managing device patients remotely.
Jose L Merino, Hospital Universitario La Paz, Madrid, Spain, analyses the implementation of actions to guarantee quality and qualification in cardiac rhythm management.
Results showed that Medtronic OptiVol Fluid Status Monitoring predicted 76% of future heart failure events as compared to only 23% detected by weight monitoring alone.
A retrospective analysis from the PROSPECT study suggests the potential for CRT to benefit more than 1 million heart failure patients worldwide.
An article published in The Lancet reports that patients with AF at risk of stroke could be offered percutaneous closure of the left atrial appendage instead of long-term warfarin therapy.
Next-generation cardiac mapping system gives physicians the ability to increase procedural efficiency and speed for treating irregular heart rhythms.
Doctors at Wake Forest University Baptist Medical Center's Heart Center have implanted one of the first wireless pacemakers in the US.
Approved to reduce the risk of cardiovascular hospitalization, Multaq will be launched this summer in the US by Sanofi-aventis.
New research reveals T-wave morphology parameters contain predictive value for mortality in the general population, independent of other clinical risk factors.
The device incorporates a micro-endoscope and light energy fibres to give physicians the capacity to see within the heart and visually direct the application of energy through a catheter.
John Camm, London, UK, analyses the first MRI approved insertable cardiac monitor (ICM) featuring an automatic AF detection algorithm.
The anticoagulant dabigatran is more effective than warfarin in the prevention of stroke in patients with atrial fibrillation, according to results from the RE-LY study.
New treatment could offer further option in addition to current standard of care in thromboembolic disease in atrial fibrillation patients.
The mortality reduction trend was also observed in high-risk patients with severely reduced heart function.
Silvia G Priori spoke to Cardiovascular News about her career, research, joy in spending time with her two children, and the occasion she took beta-blockers before meeting Dr Mike Rosen.
When did you decide you wanted a career in medicine?
In...
Riccardo Cappato, from Milan, Italy, spoke to Cardiac Rhythm News about his career, advances in cardiac rhythm management, and his passion for crime fiction.
The new sequence is in the ZFHX3 gene on chromosome 16q22, and the one third of people of European descent who carry one copy are at 20% greater risk of AF and stroke.
Boston Scientific's wireless system remotely monitors patients with implantable cardiac devices and can detects clinical events between scheduled physician visits.
Report funded by the US Agency for Healthcare Research and Quality calls for more research on the effect of catheter ablation on quality of life.
Patients with atrial fibrillation, common in those with advanced chronic heart failure, have an increased risk of hospitalisation due to heart failure.
Disorder previously believed to be benign is associated with an increased risk for atrial fibrillation, implantation of a pacemaker or death.
Latest statistics on pacemakers and implantable cardiac devices were presented at Europace 2009. Germany has one of the highest ICD implant rates in Europe.
More European countries need to embrace the remote monitoring of cardiac implantable devices, rather than offering face to face clinic visits, concluded a press briefing at Europace 2009.
Advisa DR MRI SureScan Pacing System gives pacemaker patients safe access to critical diagnostic tool.
The CABANA trial is designed to determine whether catheter ablation is more effective than drug therapy for the treatment of atrial fibrillation.
Survey shows many doctors find the management of atrial fibrillation difficult and patients are unaware of risks, complexities and treatment.
At eight years, one life is saved for every six patients who receive an ICD, according to data presented at Heart Rhythm 2009.
State-of-the-art review of catheter ablation of VT by an international panel of experts was released during the Heart Rhythm Society's 30th Annual Scientific Sessions.
OMNI study showed that one out of six patients with defibrillators to prevent sudden cardiac arrest received lifesaving medical therapy within a period of two years following their implant.
Study published in the HeartRhythm Journal reveals hidden heart health risks associated with ingredients found in diet pills.
Study in the USA will evaluate the Cardioblate Closure, a device intended to occlude the LAA permanently without introducing man-made materials into the blood stream.
Approval was granted based on select data submitted from the TOCCATA clinical study, which was performed by 17 experienced investigators at eight European centres.
Attain Ability lead is the first to use NASA technology in this kind of implantable medical device.
Analysis of data from 162 centres worldwide between 1995 and 2006 showed that tamponade caused the highest number of fatalities, followed by stroke and atrioesophageal fistula.
Dirk J van Veldhuisen analyses the findings on the use of CRT for heart failure patients classes I and II and how the results will impact in future studies.
Spectranetics laser sheath (SLS II) demonstrated 97.7% clinical success rate in removal procedures performed between January 2004 and December 2007.
Predictors of atrial fibrillation might offer physicians a better way to prevent stroke in blacks, according to a new study performed in Winston-Salem, USA.
Interactive voice response systems may help improve monitoring of patients taking anticoagulants such as warfarin while reducing the workload of clinical staff.
Drs Andrea Natale and Carlo Pappone debate the merits of magnetic (Stereotaxis) or robotic (Hansen Medical) navigation for catheter ablation of atrial fibrillation.
The landmark procedure was performed at St George's Hospital, the same London institution that undertook the first implant more than 50 years ago.
Web-based system, Merlin.net Patient Care Network combines tools that can help physicians monitor implanted cardiac devices patients and improve clinic efficiency.
Device used for closure of the left atrial appendage may be an alternative to warfarin therapy for the prevention of stroke in patients with nonvalvular atrial fibrillation.
Study shows that older men who were big during their 20s face an increased risk of suffering from atrial fibrillation.
Stanley Nattel, Montreal, Canada, spoke to Cardiac Rhythm News about his career, the challenges facing electrophysiology, and the future of the research on drugs for arrhythmias.
Cardiologist's editorial in JAMA says certified "heart electricians" should implant cardioverter defibrillators.
AnalyST Accel and Current Accel provide new alerts and insight into cardiac conditions such as atrial fibrillation and ischaemia.
Matthew Wright, Hopital Cardiologique du Haut-Levaque, Bordeaux-Pessac, France talks about techniques and developments in catheter ablation for persistent AF.
Study showed that atrial fibrillation recurred in 51.4% of patients in the drug group, as compared with 52.1% in the placebo group.
Researchers have found that delayed-enhancement magnetic resonance imaging holds promise for predicting treatment outcomes and measuring disease progression.
The St Jude Medical's Merlin.net patient care network has received a 2009 Medical Design Excellence Award.
World's smallest pacemakers feature algorithms dedicated to patient safety and proven therapies.
Boehringer Ingelheim has announced it plans to launch REAL-AF, a USA based registry to evaluate patterns in the present practice of anticoagulation in 2,500 patients.
The new Biotronik Home Monitoring integrates an intelligent traffic light system that simplifies remote monitoring of patients with implantable cardiac devices.
The Watchman, an expandable nitinol cage which blocks blood clots that typically form in the left atrial appendage, may offer alternative to blood-thinning medications.
Researchers reported that flecainide can prevent potentially lethal arrhythmias in patients with a specific type of exercise or stress-induced arrhythmia disorder called CPVT.
Study assessed the incidence of coronary artery injury early after catheter ablation for supraventricular tachycardias in children.
Study shows that knowing the level of kidney function and presence of proteinuria may improve decision making about the use of antithrombotic therapy.
Trial compares dabigatran etexilate with warfarin for the prevention of stroke and non-CNS systemic embolism in patients with atrial fibrillation.
Event to be held with the World Heart Rhythm Week, 8-14 June 2009, aims to provide information on diseases and encourage routine pulse checks.
Efficacy of the new drug was assessed in the ATHENA trial, published in the 12 February 2009 issue of the New England Journal of Medicine.
St Jude Medical's patient care network for implantable cardiac devices introduces Spanish language capabilities, new patient scheduling tools and expanded alerts for physicians.
American report says number of catheter ablation procedures for treating atrial fibrillation will fuel revenues in the electrophysiology ablation catheter market over the next five years.
The ADVANCE trial has shown that type 2 diabetes patients who had AF at the start of the study had a 61% increased risk of dying from any cause.
Program will discuss areas related to ablation, cardiac safety and a registry of procedures and outcomes from the clinical, regulatory, and industry perspectives.
A study led by investigators from Boston has demonstrated that a new immunohistochemical test is reliable in diagnosing arrhythmogenic right ventricular cardiomyopathy.
The Texas Cardiac Arrhythmia Institute, Austin, became the first in the United States to utilise the long-awaited NaviStar ThermoCool Catheter.
Researchers conducting a large study on sudden cardiac death were surprised to discover that a heart rhythm abnormality increased risk fivefold among patients with coronary artery disease.
A new study revealed that abandoning a nonfunctioning lead in an implantable cardioverter defibrillator patient is safe and does not pose a clinically significant risk of complication.
A study from the International Warfarin Pharmacogenetics Consortium confirms that using a patient's genetic information can make it easier to get the warfarin dose right.
The use of robotic navigation in procedures to treat heart rhythm problems such as atrial flutters is gaining popularity as technology becomes more and more advanced.
University of Utah researchers have developed a magnetic resonance imaging-based method for detecting and quantifying injury to the wall of the heart's left atrium in patients who have undergone a procedure to treat atrial fibrillation.
University of Utah researchers have developed a magnetic resonance imaging-based method for detecting and quantifying injury to the wall of the heart's left atrium in patients who have undergone a procedure to treat atrial fibrillation.
Investigators from The Heart Hospital, University College London, have demonstrated that atrial fibrillation ablation in older patients can be achieved safely with similar complication risks.
Headphones for MP3 players placed within an inch of pacemakers and implantable cardioverter defibrillators may interfere with these devices, according to research presented at the AHA's Scientific Sessions 2008.
New study published in the February edition of the HeartRhythm Journal confirms gender impacts risk of arrhythmias.
"Epi-needle access system" developed in the University of Virginia allows physicians to go through the fluid-filled sac to the epicardium without entering the heart.
Michel Haïssaguerre, electrophysiologist, chairman of the International Symposium on Catheter Ablation Techniques, speaks to Cardiac Rhythm News about how he started in the specialty, the French Bordeaux group and his actual research.
Why have...
Bruce Lindsay, immediate past-president of the Heart Rhythm Society, speaks to Cardiac Rhythm News about the highlights of his presidency and where he thinks the future of electrophysiology lies.
Who or what have been the...
John Camm talks about his many career achievements including his highlights, research activities and involvement in patient groups.
The novel cryoballoon device (Arctic Front, Cryocath) appears safe and effective for patients with paroxysmal atrial fibrillation (AF), but not for those with persistent AF, according to German researchers.
Results from the first trial to examine rhythm vs. rate control in heart failure patients with atrial fibrillation have been reported in the New England Journal of Medicine.
Results from a post-hoc analysis of the data from the ATHENA trial show that dronedarone reduces stroke risk in patients with atrial fibrillation.
The Corindus CorPath system, a robotic catheter manipulation platform, was discussed by Dr Peter Fitzgerald, Center for Cardiovascular Technology, Stanford University Medical Center, at the TCT meeting in October.