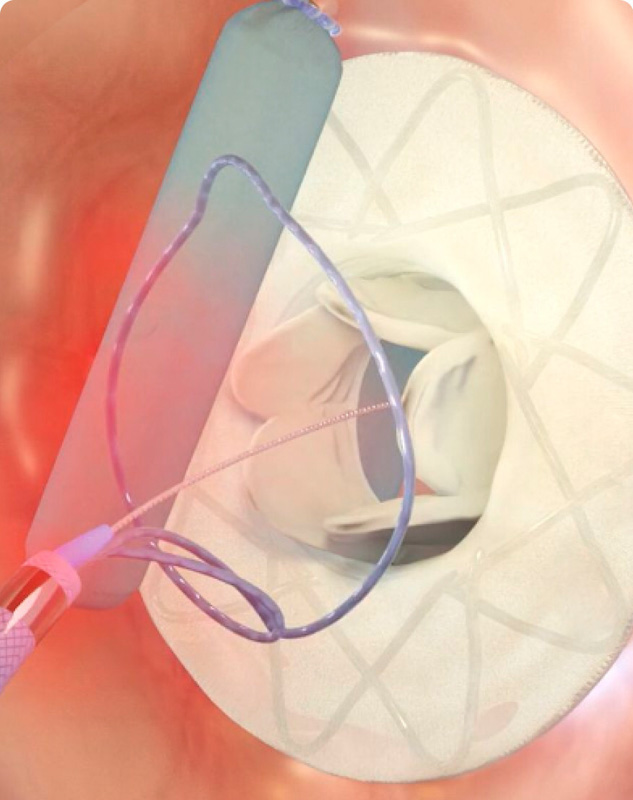
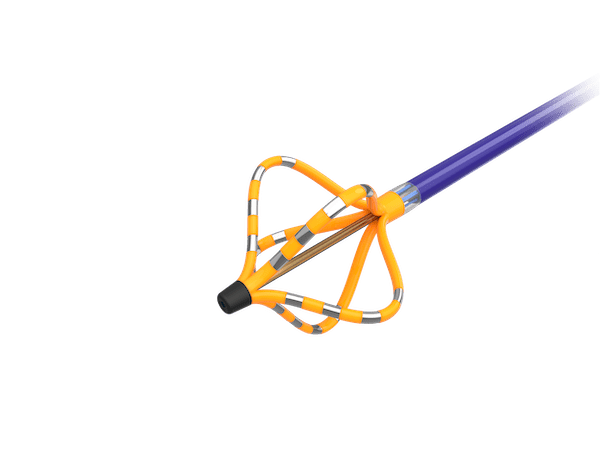
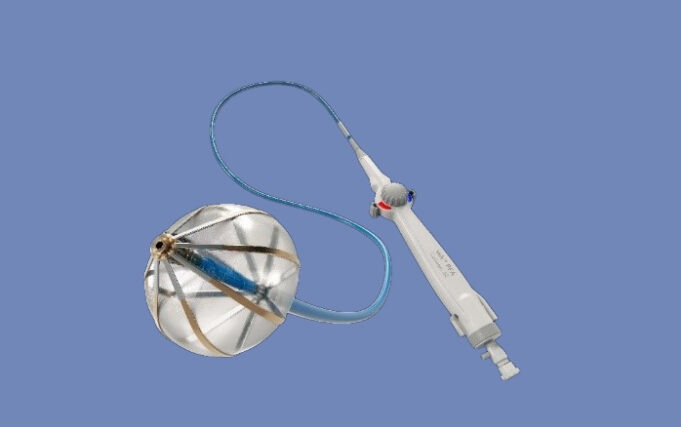
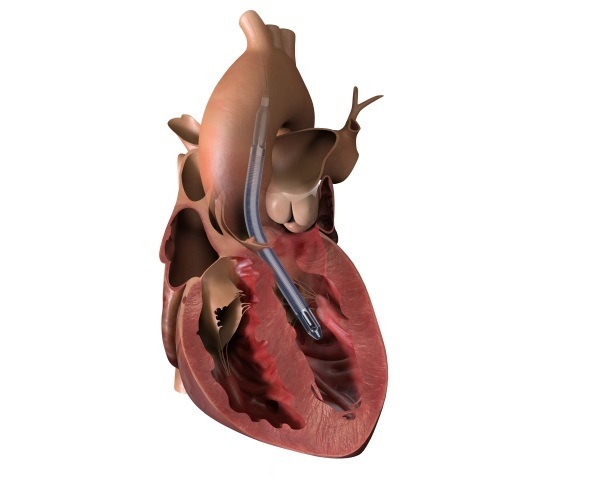
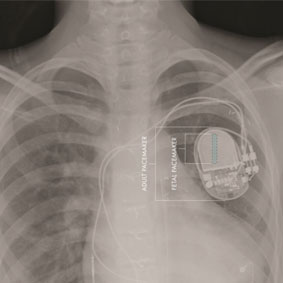
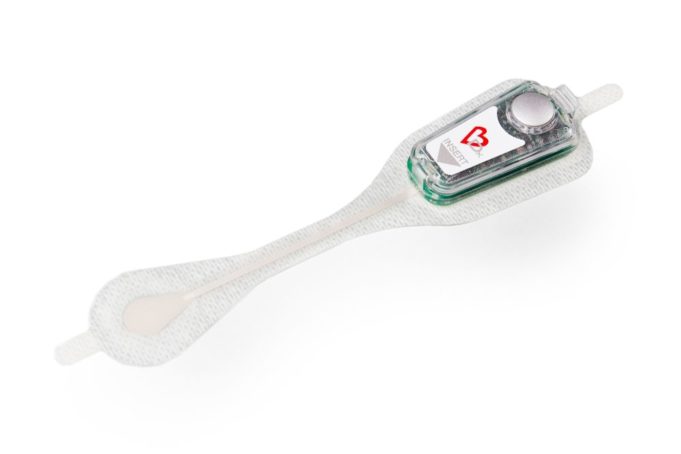
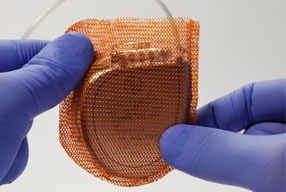
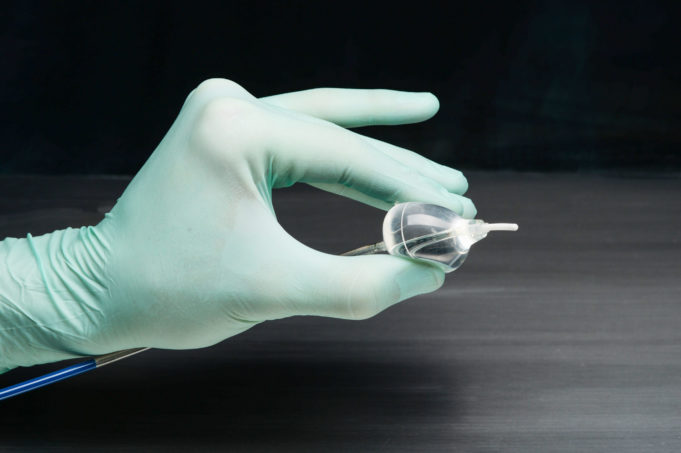
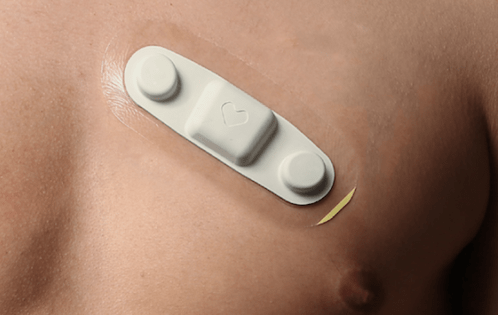
Ceryx Medical has announced the approval of a trial evaluating Cysoni-XT—a new temporary cardiac pacemaker aiming to boost recovery in heart failure.
The UK Medicines and Healthcare products Regulatory Agency (MHRA) recently approved Ceryx’s clinical trial authorisation application for the...
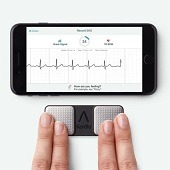
FibriCheck—a smartphone or smartwatch app capable of measuring a patient’s heart rhythm and detecting possible arrhythmias—has been cleared by the US Food and Drug Administration (FDA).
The clearance in the DHX category of smartphone electrocardiogram (ECG) devices means that US...
AtriCure has received regulatory approval from the National Medical Products Administration (NMPA) of China to market and sell several models of its AtriClip left atrial appendage (LAA) exclusion system.
The AtriClip device is designed to exclude and electrically isolate the...
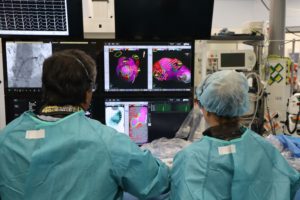
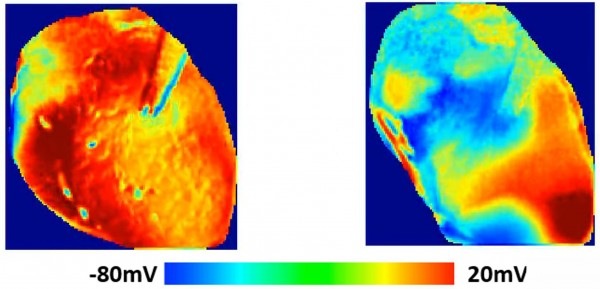
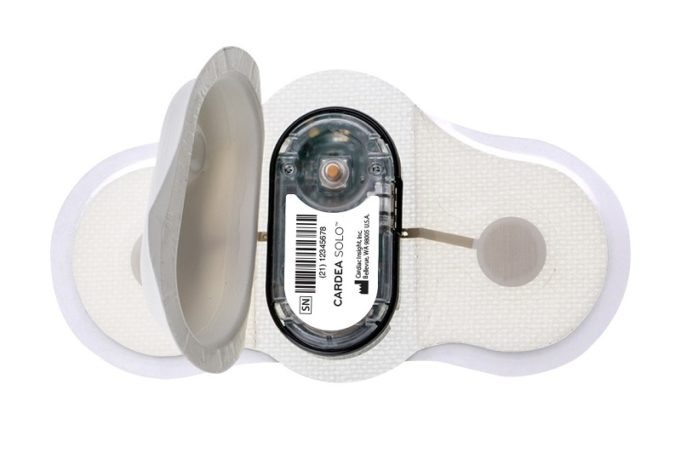
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has approved Ceryx Medical’s clinical trial authorisation application for the pilot respiratory sinus arrhythmia (RSA)-pace study of the Cysoni-XT system for post-cardiac surgery patients.
Cysoni-XT is a temporary cardiac pacemaker which...
Royal Brompton Hospital, part of Guy’s and St Thomas’ NHS Foundation Trust (London, UK) has become the first hospital in the UK to install the Magnetom Cima.X magenetic resonance imaging (MRI) system (Siemens Healthineers).
Magnetom Cima.X enables novel heart scanning...
Monitoring of heart rate and physical activity using consumer wearable devices was found to have clinical value for comparing the response to two treatments for atrial fibrillation and heart failure, the findings of a study published in Nature Medicine...
A patient in Cincinnati has become the first in the USA to receive a system that enables remote monitoring of his heart and congestion status, as well as self-adjustment of diuretics.
Vectorious Medical Technologies developed a miniature pressure sensor that...
This advertorial is sponsored by Boston Scientific
The arrival of pulsed-field ablation (PFA) has been heralded by many experts in the field of arrhythmia management as the most significant advance in the treatment of atrial fibrillation (AF) to have emerged...
This advertorial is sponsored by Biosense Webster
Biosense Webster founded the AFib Symposium in 2002. Now in its 22nd year, the annual event combines practical and scientific sessions on cutting-edge topics relevant to specialists in the treatment of atrial fibrillation...
Entries have opened for the second edition of the Global Cardiovascular Awards, celebrating innovation and excellence in the field of cardiovascular healthcare.
Returning on 13 March 2025, the second ever Global Cardiovascular Awards will feature 21 award categories recognising achievements...
Adona Medical has secured US$33.5 million in Series C financing, with the money to be used to further product development and initiate clinical use of the company’s adjustable interatrial shunt with integrated bi-atrial pressure monitoring.
The financing was led by...
Cordis recently announced that the US Food and Drug Administration (FDA) has granted premarket approval for its Mynx Control venous vascular closure device (VCD) for procedures with access sites from 6F-12F.
A press release details that the Mynx Control venous...
Pulsed field ablation (PFA) is safe for treating patients with common types of atrial fibrillation (AF), findings of the largest real-world registry of the technology to date have shown.
MANIFEST-17K is an international study that is the first to show...
Investment in a programme of clinical trials will underpin Boston Scientific’s strategy to expand the uptake of its Watchman left atrial appendage closure (LAAC) device, the company’s global vice president and general manager for the Watchman system, Angelo De...
Kardium, developer of the Globe mapping and ablation system for the treatment of atrial fibrillation (AF), has raised US$104 million in a new financing round.
The round is led by existing investor Fidelity Management & Research Company, together with follow-on...
Abbott has received CE mark in Europe for the Aveir dual chamber (DR) leadless pacemaker system.
The Aveir DR leadless pacemaker system utilises a new method of delivering dual chamber therapy as it is comprised of two unique devices—one that...
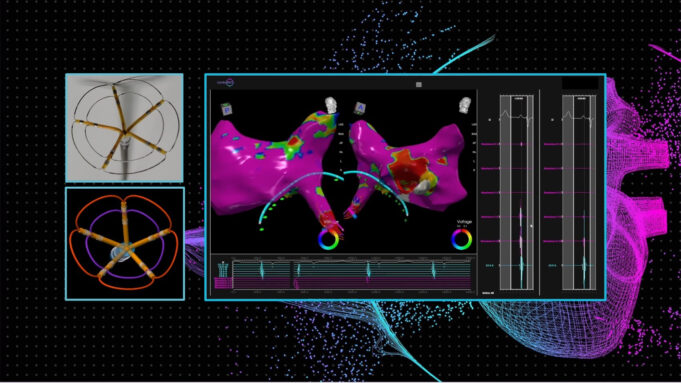
Results of the SPHERE Per-AF study, investigating the safety and efficacy of Medtronic’s Sphere-9 catheter, have been presented at the Heart Rhythm Society (HRS) annual meeting (16–19 May, Boston, USA), and have demonstrated the safety and efficacy of the...
Medtronic has announced that its investigational OmniaSecure defibrillation lead met its primary safety and effectiveness endpoints, exceeding prespecified performance goals in the global LEADR pivotal trial.
Results of the prospective, multicentre, single-arm, non-randomised, global clinical trial were presented at the...
Insights from the FARADISE registry, studying real-world outcomes from more than 1,000 patients to have undergone catheter ablation for paroxysmal and non-paroxysmal atrial fibrillation (AF) using the Farapulse pulsed field ablation (PFA) system (Boston Scientific) “reinforce the safety, efficacy...
Patients with previous paroxysmal atrial fibrillation (AF) who follow a Mediterranean diet enriched with extra-virgin olive oil after a catheter ablation procedure saw improved clinical outcomes when compared to those who freely selected their diet, research presented at the...
Six-month results from the ongoing pivotal MODULAR ATP clinical trial assessing the Boston Scientific’s mCRM modular cardiac rhythm management (CRM) system were presented at the Heart Rhythm Society (HRS) annual meeting (16–19 May, Boston, USA).
The system consists of the...
Late-breaking results from the pivotal phase of the admIRE pivotal clinical trial, alongside results from the VIRTUE study—both assessing the Varipulse (Biosense Webster) pulsed field ablation (PFA) system— were presented at the Heart Rhythm Society (HRS) annual meeting (16–19...
Atraverse Medical has announced US Food and FDA clearance of its Hotwire radiofrequency (RF) guidewire, a novel device that enables zero exchange left-heart access while also acting as a rail for catheter-based therapy systems.
This marks a significant step towards...
Adagio Medical has announced the completion of the first commercial procedures using its recently CE marked ventricular tachycardia (VT) cryoablation system and the US Food and Drug Administration (FDA) approval of the FULCRUM-VT pivotal IDE study of the VT...
Biosense Webster has announced the launch of the latest version of the its three-dimensional (3D) heart mapping system used in cardiac ablation procedures—Carto 3 system version 8.
The software features new modules including the Carto Elevate module and Cartosound Fam...
Corvia Medical has announced the publication of two-year echocardiographic data from its REDUCE LAP-HF II randomised clinical trial of the Corvia atrial shunt in JAMA Cardiology.
The study demonstrated favourable long-term effects of atrial shunting on heart structure and function...
Thermedical, a developer of advanced thermal-ablation systems to treat ventricular arrhythmias, announced today that it has completed a feasibility study utilising pulsed field ablation (PFA) therapy in combination with its SERF ablation system and Durablate catheter for treating ventricular...
Heart Rhythm Clinical and Research Solutions (HRCRS) and the Real World Evidence (RWE) Consortium have enrolled the first patient in the EVERCOOL AF study, a multicentre observational study evaluating the impact of proactive oesophageal cooling on patient outcome, throughput,...
Patients with heart failure who had an interatrial shunt inserted between the left and right atria did not see any significant benefits overall compared with those who received a placebo procedure after a median of 22 months follow-up, a...
Biotronik has announced the CE mark approval and first European implant of its latest insertable cardiac monitor (ICM)—Biomonitor IV.
The device features artificial intelligence (AI) for false positive reduction, and according to Biotronik is the only ICM on the market...
Three-month follow-up results from the SmartfIRE clinical trial, investigating Biosense Webster’s Thermocool Smarttouch SF dual energy catheter were presented in a late-breaking trial session at the European Heart Rhythm Association (EHRA) congress (7–9 April, Berlin, Germany).
Tom De Potter, an...
Clinical findings of the B3 study, assessing the impact of closed loop stimulation on the incidence of sub-clinical atrial fibrillation (AF) in sinus node disease patients, have been presented during a late-breaking trial session at the European Heart Rhythm...
Interim first-in-human clinical safety and efficacy results for Medtronic’s Sphere-360 investigational single-shot mapping and ablation catheter using pulsed field (PF) energy, for treatment of patients with paroxysmal atrial fibrillation (AF) were presented as a late-breaking clinical trial at the...
A randomised trial investigating preventive ablation of a potential arrhythmogenic substrate associated with coronary chronic total occlusion (CTO) in patients at high risk of ventricular arrhythmias (VAs) has found that this approach may reduce the risk of appropriate implantable...
A trial of technology using ultra-low temperature cryoablation (ULTC) has shown that the technique eliminated clinical ventricular tachycardia (VT) in 94% of patients.
The finding of the Cryocure-VT trial, which involved the use of Adagio Medical’s vCLAS catheter, were presented...
An international consensus statement on how to treat atrial fibrillation (AF) with catheter or surgical ablation has been published today in EP Europace and presented at the European Heart Rhythm Association (EHRA) 2024 congress (7–9 April, Berlin, Germany).
Lead author...
Boston Scientific has initiated the NAVIGATE-PF study of the Faraview software module when it is used to visualise and track the Farawave Nav pulsed field ablation (PFA) catheter for the treatment of patients with paroxysmal and persistent atrial fibrillation...
Automated external defibrillators (AEDs) were used in in 13 of nearly 1,800 cases of out-of-hospital cardiac arrest, even though many of the incidents occurred near a public AED, research to be presented at the American College of Cardiology’s (ACC’s)...
GE HealthCare has announced the launch of Caption AI artificial intelligence (AI)-driven software for Vscan Air SL, its dual-headed, handheld, wireless, point-of-care ultrasound imaging system.
With the addition of Caption AI, clinicians using Vscan Air SL handheld ultrasound will have...
Kardium has announced the successful completion of enrolment in the paroxysmal cohort of the pivotal PULSAR IDE (investigational device exemption) study of the Globe pulsed field ablation (PFA) system for the treatment of atrial fibrillation (AF).
This marks a milestone...
Viz.ai has announced a strategic collaboration with Medtronic to improve the coordination of cryptogenic stroke patient care between neurology and cardiology teams.
For stroke patients who are at risk of atrial fibrillation (AF) post-stroke and may need additional cardiac monitoring,...
High-power short-duration ablation results in significantly shorter procedure times without affecting procedural efficacy and safety among patients undergoing ablation for atrial fibrillation (AF).
This is according to the findings of a single-centre study investigating the impact of high-density mapping alongside...
Biosense Webster has submitted a premarket approval application (PMA) to the US Food & Drug Administration (FDA) for its Varipulse platform. The submission was supported by results from the admIRE study, a prospective, multicentre, non-randomised trial.
The Varipulse platform is...
Adagio Medical has announced CE mark approval of its ultra-low temperature cryoablation (ULTC) system for the treatment of monomorphic ventricular tachycardia.
The system consists of the upgraded cryoablation console, also capable of supporting atrial ablation procedures using commercially available iCLAS...
Abbott has announced it has received CE mark in Europe for its Assert-IQ insertable cardiac monitor (ICM).
Physicians can insert the Assert-IQ ICM under the skin of the chest to monitor a person's heart continuously and detect arrhythmias. After insertion,...
Following the recent CE approval of the Solia S lead for left bundle branch area pacing (LBBAP), Biotronik has announced the first complete conduction system pacing (CSP) system, now fully CE-approved for LBBAP.
The Biotronik CSP system comprises three components:...
People at risk should be tested for atrial fibrillation (AF) every time they attend a health appointment, according to results of the AFFECT-EU project. Patients at high risk of the disorder, such as those with heart failure or prior...
HeartBeam has announced that it has enrolled the first patients in the VALID-ECG pivotal study. The first patients were enrolled at Atlanta Heart Specialists (Atlanta, USA).
The VALID-ECG study will evaluate the performance of a 12-lead electrocardiogram (ECG) synthesised from...
The US Food and Drug Administration (FDA) has approved an additional indication for the weight loss drug semaglutide—marketed as Wegovy (Novo Nordisk) —to reduce the risk of major cardiovascular events such as death, heart attack, or stroke in adults...
Stereotaxis has announced that regulatory submissions were made recently in both Europe and the USA for the MAGiC catheter. These submissions follow initial clinical results in an ongoing trial.
Stereotaxis’ MAGiC catheter is a robotically navigated magnetic ablation catheter designed...
Haemonetics Corporation has entered into a definitive agreement to acquire Attune Medical, the manufacturer of the ensoETM proactive oesophageal cooling device, the only US Food and Drug Administration (FDA)-cleared temperature regulation device indicated for oesophageal protection during radiofrequency (RF)...
Six-month results from the PROACTIVE-HF pivotal trial, evaluating the Cordella pulmonary artery (PA) sensor (Endotronix) in New York Heart Association (NYHA) class III heart failure (HF) patients at risk of congestion, show that the trial met primary safety and...
BioCardia has announced interim results from the phase III randomised controlled trial of its CardiAMP autologous cell therapy in 110 randomised patients with advanced chronic heart failure at a mean 20-month follow-up, (CardiAMP HF).
Results showed reductions in heart death...
Volta Medical has announced CE mark for its Volta AF-Xplorer, an artificial intelligence (AI) companion that is cleared in the USA for the real-time manual or automatic annotation of spatiotemporal dispersions during AF and atrial tachycardia (AT) procedures.
Volta’s AF-Xplorer...
Biosense Webster has announced the receipt of European CE mark approval for the Varipulse platform for the treatment of symptomatic drug refractory recurrent paroxysmal atrial fibrillation (AF) using pulsed field ablation (PFA).
The Varipulse platform is comprised of the Varipulse...
FIRE1 today announced that it has completed patient enrolment in the US early feasibility study—FUTURE-HF2—of its FIRE1 system for remote heart failure monitoring.
The FIRE1 system is designed to directly measure a patient’s volume status by measuring the inferior vena...
A scientific statement published by the American Heart Association (AHA) in Circulation today acknowledges the potential “transformative” impact of the technology on cardiovascular medicine, but challenges remain in its development, the authors of the paper state.
The new scientific...
Researchers have detailed how artificial intelligence (AI) can be used to predict sudden cardiac arrest by studying patterns in electrocardiograms (ECGs) as well as differentiating between pulseless electrical activity and ventricular fibrillation.
The findings from researchers at Cedars-Sinai Health System...
VahatiCor has announced the treatment of the first patient with the A-Flux Reducer system, an interventional treatment for patients with angina or chest pain.
The implant is placed in the coronary sinus and is designed to provide more blood flow...
Biosense Webster has announced the commencement of patient cases with the investigational Laminar left atrial appendage elimination (LAAX) system as part of its pivotal investigational device exemption (IDE) study.
The first procedures, performed by Saibal Kar (Los Robles Health System,...
FineHeart has announced the successful filing of six new international patents for FlowMaker, a fully implantable device for the treatment of patients with advanced heart failure.
These patents increase the company’s international portfolio to 147 within 25 patent families, covering...
BiVACOR, a clinical-stage medical device company, has announced that US$13 million has been awarded from the Australian government’s Medical Research Future Fund (MRFF) grant through the Artificial Heart Frontiers Program (AHFP) to support BiVACOR’s total artificial heart programme and...
Reprieve Cardiovascular, a development stage company focused on pioneering an automated diuretic and fluid management approach for acute decompensated heart failure (ADHF) treatment, announced it has raised a total of US$42 million in series A financing.
The total round was...
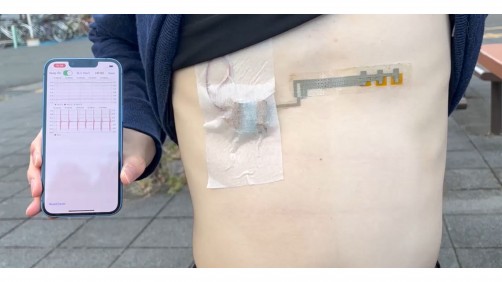
X-trodes has announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for the company’s Smart Skin solution, marketed as X-trodes System M, a wireless wearable technology for advanced electrophysiological monitoring.
X-trodes’ Smart Skin is comprised of...
A person’s chance of surviving while receiving cardiopulmonary resuscitation (CPR) for cardiac arrest in hospital declines rapidly from 22% after one minute to less than 1% after 39 minutes, the findings of a US study published in The BMJ...
Pulse Biosciences has announced favourable findings from the 60-day post-procedure evaluations for four initial patients treated in the Company’s CellFX nanosecond pulsed field ablation (nsPFA) 360° cardiac catheter first-in-human feasibility study.
“The 60-day remap results for the initial patients treated...
This advertorial is sponsored by Biosense Webster
The field of catheter ablation for atrial fibrillation (AF) is evolving. The development of pulsed field ablation (PFA), as well as advances in established technologies such as radiofrequency (RF) ablation, help catheter ablation...
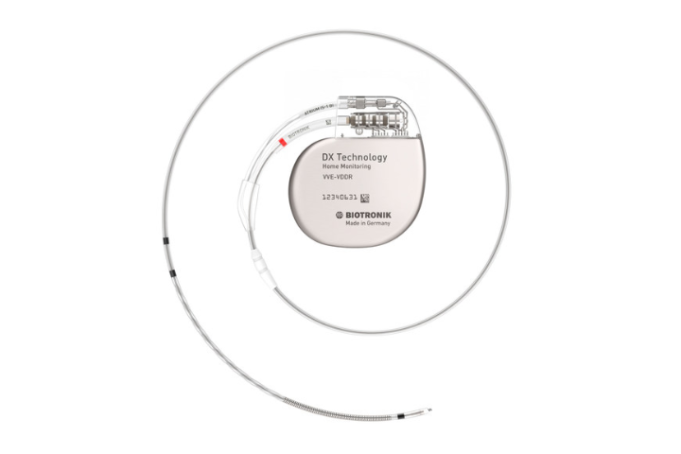
Biotronik has announced that it will solely supply the proprietary DX models for new single-chamber implantable cardioverter-defibrillators (ICDs) implants moving forward.
The move is being made in response to overwhelming recent clinical data demonstrating superior diagnostics and decreased complication risk...
Biosense Webster has announced support for two collaborative studies, VIRTUE and POLARIS aiming to better understand the use and workflows with the investigational Varipulse pulsed field ablation (PFA) platform for treating patients with diverse arrhythmias.
The VIRTUE study is evaluating...
Medtronic has announced new cost-effectiveness data from the STROKE AF clinical study, which showed that continuous monitoring with the Reveal LINQ insertable cardiac monitor (ICM) is significantly more cost-effective than the standard of care in ischaemic stroke patients with...
Twelve-month results from the inspIRE study, investigating predictors of success for pulmonary vein isolation with pulsed field ablation (PFA) using a variable loop catheter with 3D mapping integration have been presented as a late-breaking presentation at AF Symposium 2024...
Posterior wall ablation, a commonly used adjunct to pulmonary vein isolation (PVI), does not add any benefits for patients undergoing catheter ablation for persistent atrial fibrillation (AF), late-breaking research presented at AF Symposium 2024 (1–3 February, Boston, USA) has...
Findings of an investigation into whether ablation of non-pulmonary vein atrial fibrillation (AF) drivers identified by machine learning in addition to pulmonary vein isolation improves procedural outcomes have been presented at the AF Symposium 2024 (1–3 February, Boston, USA).
The...
Data from the first US comprehensive single-centre experience at St Bernard’s Medical Center and Arrhythmia Research Group (Jonesboro, USA), presented at a late-breaking session at the AF Symposium 2024 (1–3 February, Boston, USA) shows that left atrial appendage occlusion...
Boston Scientific has received US Food and Drug Administration (FDA) approval for the Farapulse pulsed field ablation (PFA) system.
The Farapulse PFA system is indicated for the isolation of pulmonary veins in the treatment of drug-refractory, recurrent, symptomatic, paroxysmal atrial...
Biotronik has announced the milestone achievement of 100,000 implanted single-chamber implantable cardioverter-defibrillators (ICDs) equipped with DX Technology.
Since the introduction of the technology in 2009, clinicians in more than 80 countries across all continents use DX ICDs.
“Reaching this significant milestone,...
Element Science has announced that it has received CE mark certification and UK Conformity Assessed (UKCA) marking for its novel patch-wearable cardioverter defibrillator (P-WCD) from its notified body, the BSI Group.
The Jewel P-WCD was designed to address limitations with...
The full shortlist for the inaugural Global Cardiovascular Awards—which will recognise the tireless work of individuals, teams and organisations to improve the life of sufferers of cardiovascular disease—has now been revealed.
An expert panel of judges has hand-picked finalists and...
Tributes have poured in from across the world of cardiology following the death of Bruce Wilkoff, a pioneer of cardiac electrophysiology and former president of the Heart Rhythm Society (HRS), who passed away early in January aged 69.
Wilkoff had...
Abbott has announced the first global procedures have been conducted using the company’s new Volt pulsed field ablation (PFA) system to treat patients with arrhythmias such as atrial fibrillation (AF).
Over 30 patients were recently treated in Australia as part...
Cardiac Dimensions, a developer of invasive treatment modalities to address patients suffering from heart failure with functional mitral regurgitation (FMR), has announced that interventional cardiologist Satya Shreenivas has joined the company as chief medical officer.
Shreenivas has led the structural...
Occlutech has announced that the US Food and Drug Administration (FDA) has approved the Occlutech ASD Occluder and Occlutech Pistol Pusher for the treatment of atrial septal defects (ASD).
With this approval, Occlutech will immediately begin commercialisation in an exclusive...
Orchestra BioMed Holdings has announced the first patient was randomised in the BACKBEAT pivotal study in late December 2023.
The BACKBEAT pivotal study will evaluate the efficacy and safety of atrioventricular interval modulation (AVIM) therapy (also known as BackBeat CNT),...
CardioFocus has announced the acquisition of Galvanize Therapeutics' Electrophysiology Technology division.
Notable assets included in the acquisition are the Centauri system pulsed electric field generator, which is CE marked and commercially available in the EU and the UK, and the...
People taking medical cannabis for chronic pain have a slightly increased risk of arrhythmia, according to research published in the European Heart Journal.
Recreational use of cannabis has been linked to cardiovascular disease but there has been very little research...
Biosense Webster has today announced approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for the Varipulse platform for the treatment of symptomatic drug refractory recurrent paroxysmal atrial fibrillation (AF) using pulsed field ablation (PFA).
The Varipulse platform...
GE HealthCare has entered into an agreement to acquire MIM Software, a provider of artificial intelligence (AI)-enabled image analysis and workflow tools across multiple care areas, including oncology, urology, neurology, and cardiology
GE HealthCare expects to leverage MIM Software’s imaging...
Stereotaxis has announced that the first patients have been successfully treated using its Magnetic Interventional Ablation Catheter, MAGiC.
Stereotaxis’ MAGiC catheter is a robotically navigated magnetic ablation catheter designed to perform minimally invasive cardiac ablation procedures. The first human procedures...
Medtronic has received CE mark for its Micra AV2 and Micra VR2, the next generation of its miniature, leadless pacemakers.
Micra AV2 and Micra VR2, the world’s smallest pacemakers, provide longer battery life and easier programming than prior Micra pacemakers,...
Endotronix has submitted a premarket approval (PMA) application for its Cordella pulmonary artery (PA) sensor system to the US Food and Drug Administration (FDA).
Cordella is a heart failure patient management platform that delivers proactive PA pressure data and non-invasive...
iRhythm Technologies has announced that its long-term ambulatory cardiac monitor, the Zio monitor ECG system, has received CE mark certification under the European Union’s Medical Device Regulation (MDR) from its notified body, the BSI Group.
The Zio monitor ECG system...
Pulse Biosciences has filed a premarket notification 510(k) to the US Food and Drug Administration (FDA) for its novel CellFX nanosecond pulsed field ablation (nsPFA) cardiac clamp.
Pursuant to Section 510(k), once the application has been accepted, the FDA will...
Allowing patients to eat before elective cardiac catheterisation posed no safety risk and benefited patient satisfaction and overall care, research published in the American Journal of Critical Care has shown.
Patients undergoing coronary artery catheterisation are typically required to take...
Boston Scientific has initiated the AVANT GUARD clinical trial to evaluate the safety and effectiveness of the Farapulse pulsed field ablation (PFA) system as a first-line treatment for persistent atrial fibrillation (AF), the only trial to study the use...
CardioRenal and CHU Grenoble Alpes have announced an innovation that allows chronically ill patients to measure their blood potassium levels autonomously and accurately.
The Tenor device enables patients to measure their blood potassium levels at home, similar to how diabetic...
LUMA Vision, developer of a novel four-dimensional (4D) cardiac imaging and navigation platform, has announced a total of US$22 million in new financing for the company.
The Series A3 financing added three new investors comprising an undisclosed multinational strategic investor,...
Biosense Webster has announced the first patient cases with the investigational dual energy Thermocool Smarttouch SF catheter took place as part of the SmartPulse pivotal study for treatment of paroxysmal atrial fibrillation (AF).
The first procedures were performed by David...
Which stories captured the attention of the electrophysiology community across 2023? Read our summary of the trending stories from across the Cardiac Rhythm News network throughout the year.
What were your highlights? Leave a reply in the comment box at...
Results of the National Cardiogenic Shock Initiative—a single-arm multicentre study assessing the feasibility and effictiveness of utilising early mechanical circulatory support using the Impella (Abiomed) device in patients presenting with acute myocardial infarction complicated by cardiogenic shock—have demonstrate a...
Individuals infected with COVID-19 are also at an increased risk of suffering from cardiac arrhythmias, research published online in European Heart Journal Open has concluded.
The authors of the study, Ioannis Katsoularis (University Hospital of Northern Sweden, Umeå, Sweden) and...
Smartwatches can help physicians detect and diagnose irregular heart rhythms in children, research published online in Communications Medicine has shown.
The finding comes from a survey of electronic medical records for paediatric cardiology patients receiving care at Stanford Medicine Children’s...
Medtronic has announced that the US Food and Drug Administration (FDA) has approved the PulseSelect pulsed field ablation (PFA) system for the treatment of both paroxysmal and persistent atrial fibrillation (AF).
This is the first PFA technology to receive FDA...
CathVision has announced the launch of ECGenius 3.1, an advanced version of its ECGenius system software.
The new version improves workflow and streamlines processes to accelerate adoption as electrophysiologists integrate artificial intelligence (AI) analyses into the electrophysiology (EP) lab, increasing...
Cardiovascular diseases, including ischaemic heart disease, stroke, heart failure, peripheral and aortic diseases, arrhythmias and valvular heart disease remain the leading global cause of death, and a major contributor to health loss worldwide, new data published in the Journal...
The cardiologist and researcher, Harlan M Krumholz (Yale School of Medicine, New Haven, USA), has been named editor-in-chief of the Journal of the American College of Cardiology (JACC), the flagship journal of the American College of Cardiology.
“The opportunities ahead...
Medtronic has announced the launch of its Penditure left atrial appendage (LAA) exclusion system in the USA.
The Penditure LAA Exclusion System is an implantable clip that comes pre-loaded on a single-use delivery system for use in left atrial appendage...
Procyrion has announced the enrolment of the first patients in the company’s investigational device exemption (IDE) pivotal trial.
The DRAIN-HF study will evaluate the Aortix percutaneous mechanical circulatory support (pMCS) technology in patients with acute decompensated heart failure (ADHF) who...
The American College of Cardiology (ACC) and the American Heart Association (AHA), with several other medical associations, have issued new guidelines for the prevention and management of atrial fibrillation (AF). The guideline was jointly published in the Journal of...
Johnson & Johnson MedTech has announced the completion of the acquisition of Laminar, a medical device company focused on eliminating the left atrial appendage (LAA) in patients with non-valvular atrial fibrillation (AF).
Johnson & Johnson MedTech acquired Laminar for...
Healthcare 21 (HC21) has gained national reimbursement for Catheter Precision’s VIVO 3D mapping system in the UK following it being granted inclusion in National Health Service (NHS) England's Specialised Services Devices Programme (SSDP).
NHS England’s SSDP, previously known as High-Cost...
Abiomed has announced that the first patient in the world has been enrolled in the landmark RECOVER IV randomised controlled trial (RCT).
The on-label, two-arm trial will randomise 548 patients to assess whether Impella support prior to percutaneous coronary intervention...
Ischaemic strokes in atrial fibrillation (AF) patients are less often disabling or fatal if they have been treated with left atrial appendage closure procedure (LAAC) compared to being treated with direct oral anticoagulants (DOACs), research published in JACC: Clinical...
The occurrence of atrial fibrillation (AF) after mitral valve surgery may be more harmful than previously thought, the authors of a research paper in the Journal of Thoracic and Cardiovascular Surgery (JCTVS) have stated.
Whitney Fu (University of Michigan Health...
A clinical trial to challenge the routine implantation of a defibrillator in myocardial infarction (MI) survivors with heart failure has enrolled its first patient. The PROFID EHRA trial is part of the EU-funded PROFID project, which aims to personalise...
Pulse Biosciences has announced the filing of a premarket notification 510(k) to the US Food and Drug Administration (FDA) for its novel CellFX nanosecond pulsed field ablation (nsPFA) percutaneous electrode.
The company’s percutaneous electrode is an image-guided needle designed to...
Medtronic has received CE mark for the PulseSelect pulsed field ablation (PFA) system and the Nitron CryoConsole.
The PulseSelect system is designed to treat atrial fibrillation (AF) effectively, efficiently, and safely, with a new ablation modality that uses pulsed electric...
Element Science has announced the successful completion and presentation of the results of the Jewel investigational device exemption (IDE) study, assessing the Jewel patch-wearable cardioverter defibrillator (P-WCD).
The study, which enrolled 305 patients, marks a significant milestone in the field...
Abbott has announced new late-breaking data that show advanced heart failure patients living with its HeartMate 3 left ventricular assist device (LVAD) who did not receive aspirin experienced fewer complications from bleeding and were associated with reduced hospital visits...
Analysis of outcomes of more than 17,000 patients receiving pulsed field ablation (PFA) using the Farapulse (Boston Scientific) device reinforce the safety profile of the system, investigators say, with no reported permanent phrenic nerve palsy, pulmonary vein stenosis or...
RCE Technologies, the developer of a non-invasive, instant measurement of cardiac proteins was selected as the winner of the annual Health Tech competition at the 2023 American Heart Association (AHA) Scientific Sessions (11–13 November, Philadelphia, USA).
The winning technology focuses...
Use of apixaban in patients with sub-clinical atrial fibrillation (AF) resulted in a lower risk of stroke or systemic embolism than aspirin, but a higher risk of major bleeding, results of the ARTESIA randomised trial, presented at the American...
Results of the SELECT clinical trial, presented at the American Heart Association’s 2023 Scientific Sessions (11–13 November, Philadelphia, USA), have shown that overweight or obese people without diabetes taking the drug semaglutide for more than three years had a...
Conformal Medical has announced positive one-year results from the company's CONFORMAL Early Feasibility Study (EFS).
William Gray (Lankenau Heart Institute, Wynnewood, USA) presented the Conformal CLAAS left atrial appendage occlusion (LAAO) device EFS one-Year TEE follow-up during a moderated abstract...
Patients with atrial fibrillation (AF) undergoing a transcatheter aortic valve implantation (TAVI) at the same time as a left atrial appendage occlusion (LAAO) procedure using the Watchman (Boston Scientific) device had similar outcomes when compared to patients getting TAVI...
Implicity has announced the results of a clinical study published in the Cardiovascular Digital Health Journal, reaffirming that its proprietary algorithm is highly effective at identifying patterns and classifying AF episodes into medically relevant events that require clinical action,...
Women with atrial fibrillation (AF) undergoing pulsed field ablation (PFA) have just as good outcomes as men according to a large-scale international study published in JAMA Cardiology.
This study is the first to compare sex outcomes for AF patients undergoing...
Biosense Webster has announced new findings from the Q-FFICIENCY study have been published in the Journal of Cardiovascular Electrophysiology, which the company says demonstrate improved control of atrial fibrillation (AF), relief of symptoms and overall quality of life, following...
Medtronic has received US Food and Drug Administration (FDA) approval for the Aurora EV-ICD MRI SureScan (extravascular implantable cardioverter-defibrillator) and Epsila EV MRI SureScan defibrillation lead to treat dangerously fast heart rhythms that can lead to sudden cardiac arrest (SCA).
The...
A research article in the journal Science has found that fibroblasts—scar-forming cells that reside in the scar tissue of an injured heart—directly play a role in promoting arrhythmia.
This finding holds promise for novel approaches to life threatening rhythm problems...
FEops has entered into a partnership with ConcertAI’s TeraRecon for the commercialisation of FEops HEARTguide’s for left atrial appendage (LAA) occlusion workflow, with a focus on the US market.
“We are thrilled to join forces with TeraRecon and integrate our...
Endotronix has received investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) for a subsequent multicentre study, PROACTIVE-HF 2, which will evaluate the company's Cordella sensor for pulmonary artery (PA) pressure-guided therapy.
The prospective, dual-arm trial...
This advertorial is sponsored by Boston Scientific
Physicians at Na Homolce Hospital (Prague, Czech Republic) have recently completed their 2,000th clinical case using the FARAPULSE (Boston Scientific) pulsed field ablation (PFA) system. The completion of the procedure marks an important...
Pulse Biosciences has announced a collaboration with CardioNXT, to support the company’s planned nanosecond pulsed field ablation (nsPFA) cardiac catheter first-in-human study focused on the treatment of atrial fibrillation (AF).
“We are excited to announce this collaboration and the progress...
Boston Scientific today launched the LUX-Dx II+ insertable cardiac monitor (ICM) system, describing it as a next-generation insertable monitor for long-term monitoring of arrhythmias associated with conditions such as atrial fibrillation (AF), cryptogenic stroke and syncope.
The system is designed...
Anumana has announced US Food and Drug Administration (FDA) 510(k) clearance for ECG-AI LEF, a breakthrough artificial intelligence (AI)-powered medical device to detect low ejection fraction (LEF) in patients at risk of heart failure.
“Anumana’s ECG-AI LEF fills an important...
This advertorial has been sponsored by GE HealthCare.
In this white paper Usman Siddiqui (Advent Health, Orlando, USA) discusses the reasons why the Prucka 3 with CardioLab Digital Recording System stands out among electrophysiology labs.
Featuring a robust ecosystem encompassing...
Research published in JACC: Clinical Electrophysiology has found a significant reduction in the rate of atrioesophageal fistuals (AEFs) during cardiac ablation of the left atrium when with the use of the ensoETM (Attune Medical) system when compared luminal oesophageal...
Medtronic has announced the results from two analyses demonstrating survival benefits and a reduction in life-threatening cardiac events with the use of implantable cardioverter defibrillators (ICDs).
The data provide real-world evidence supporting the use of Medtronic ICDs for indicated patients,...
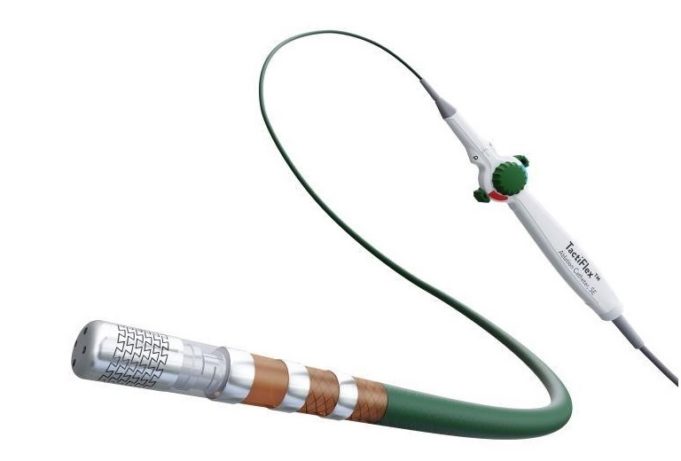
Abbott has announced that the company's TactiFlex ablation catheter has been used for the first time in Canada at the Foothills Medical Centre in Calgary, to treat atrial fibrillation (AF).
The company describes the device as the world's first ablation...
Field Medical has closed its oversubscribed seed round with investments totalling US$14million.
The convertible note funding was led by private investors who were joined by multiple strategic investors. This financing will support preclinical-to-clinical development activities including first-in-human studies planned for...
Anthos Therapeutics has announced that the AZALEA-TIMI 71 Phase 2 study has been stopped early due to an “overwhelming” reduction in the composite of major and clinically relevant non-major bleeding in patients taking abelacimab compared to patients taking rivaroxaban.
The...
Biosense Webster has announced that the first cases have been performed using the investigational Omnypulse catheter as part of the Omny-IRE clinical trial. The first procedures were performed by Mattias Duytschaever at AZ Sint-Jan Hospital in Brugge, Belgium.
The Omny-IRE...
Younger atrial fibrillation (AF) patients are most likely to benefit from more personalised, magntetic resonance imaging (MRI)-guided ablation, according to a new analysis of the DECAAF II trial using artificial intelligence (AI). This was the conclusion of research presented...
Switching vitamin K antagonist (VKA) treatment to a non-vitamin K antagonist oral anticoagulant (NOAC) in frail elderly patients with atrial fibrillation (AF) is associated with more bleeding complications compared to continuing VKA treatment.
This is according to the findings of...
SmartCardia has received US Food and Drug Administration (FDA) clearance for its seven-lead real-time electrocardiogram (ECG) monitoring patch and cloud platform.
SmartCardia's 7L patch is cable-free, waterproof and can be used for continuous monitoring for up to 14 days.
“SmartCardia's 7L...
Adagio Medical has announced the first ultra-low temperature cryoablation (ULTC) procedure performed using the Adagio vCLAS catheter system in the USA as part of the FULCRUM-VT clinical trial. The procedure was performed at Banner - University Medical Center (Phoenix,...
Attune Medical has been granted de novo marketing authorisation from the US Food and Drug Administration (FDA) for its ensoETM device to reduce the likelihood of ablation-related oesophageal injury resulting from radiofrequency cardiac ablation procedures.
The FDA based its decision...
Stereotaxis has announced that physicians at Heart Centre Rigshospitalet of Copenhagen University Hospital successfully treated the first patients using Stereotaxis’ Genesis robotic magnetic navigation system. Rigshospitalet is among the first in Europe, and the only hospital in Denmark, to...
Vektor Medical has announced the release of a series of software enhancements to its AI-based non-invasive solution, vMap.
Designed to improve ablation outcomes and procedural efficiencies, the newly updated vMap software integrates additional automation and advanced visualisation features, the company...
Boston Scientific has received US Food and Drug Administration (FDA) approval for the latest-generation Watchman FLX Pro left atrial appendage closure (LAAC) device.
The device, which is indicated to reduce stroke risk in patients with non-valvular atrial fibrillation (NVAF) who...
Atrial fibrillation (AF) ablation is associated with lower rates of death, urgent heart transplantation or left ventricular assist device (LVAD) implantation compared with medical therapy in patients with end-stage heart failure, research presented in at the European Society of...
Biotronik has announced the first US implantation of the Amvia Edge pacemaker. The procedure was successfully completed by Raul Weiss at Mount Sinai Medical Center in Miami, Florida.
Amvia Edge is Biotronik’s next-generation pacemaker family, featuring MRI Guard 24/7, a...
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has designated three new approved bodies to increase the country’s capacity to certify medical devices.
TÜV SÜD, Intertek, and TÜV Rheinland UK join the four current UK Approved Bodies, almost doubling...
Results of the first randomised trial comparing pulsed field ablation (PFA) to thermal ablation for the treatment of paroxysmal atrial fibrillation (AF)—ADVENT—have shown that PFA is as safe and effective at 12 months.
Findings of the study, which involves Boston...
GE Healthcare has announced the launch of two new tools for the cardiology market, coinciding with the European Society of Cardiology (ESC) congress (25–28 August, Amsterdam, The Netherlands).
The launches include Vscan Air SL, a dual-headed, handheld, wireless ultrasound imaging...
Oral anticoagulation in patients with atrial high rate episodes (AHRE) increases bleeding without reducing a composite outcome of stroke, systemic embolism or cardiovascular death.
This is the headline finding from the NOAH-AFNET 6 trial, presenting during a hot line trial...
Physical fitness is linked with a lower likelihood of developing atrial fibrillation (AF) and stroke, a study of more than 15,000 people, to be presented at the European Society of Cardiology (ESC) congress (24–28 August, Amsterdam, The Netherlands), has...
The British Heart Foundation (BHF) has appointed research leader and NHS consultant physician Bryan Williams as its first chief scientific and medical officer.
Williams will join the charity’s executive group in Autumn 2023 and succeed Sir Nilesh Samani (medical director)...
AstraZeneca’s Forxiga (dapagliflozin) has been approved in China for use in adults with symptomatic chronic heart failure.
The drug has previously been approved in China for heart failure patients with reduced ejection fraction (HFrEF) meaning that Forxiga is now approved...
Heart failure start-up Acorai has announced the initiation of a global clinical trial with the enrolment of its first patient in London, UK.
The Acorai heart monitor was designated as a breakthrough device by the US Food and Drug Administration...
An artificial intelligence (AI) model may be more efficient at detecting signatures of atrial septal defect (ASD) in electrocardiograms (ECG) than traditional methods.
This is according to investigators from Brigham and Women’s Hospital (Boston, USA) and Keio University (Tokyo, Japan),...
Research published in the journal Circulation: Cardiovascular Quality and Outcomes has highlighted the small number of clinical trials in cardiovascular medicine currently conducted in Africa.
The authors of the research letter, writing from Egypt and the USA, say that though...
CathVision has announced US Food and Drug Administration (FDA) clearance and commercial availability of the PVI Analyzer and Signal Complexity algorithms, part of the Cardialytics suite of artificial intelligence-powered analytics integrated into the ECGenius system.
According to a CathVision press...
Viz.ai has received a de novo approval by the US Food and Drug Administration (FDA) for its Viz HCM module, a hypertrophic cardiomyopathy (HCM) artificial intelligence (AI) detection algorithm.
The deployment of the algorithm is financially supported by a multi-year...
Ian Meredith is a well-known figure within the field of interventional cardiology, not only for a distinguished academic and clinical career but also his six-year spell as the executive vice president and global chief medical officer at Boston Scientific.
After...
Boston Scientific has received US Food and Drug Administration (FDA) approval for the POLARx cryoablation system.
The new system, which is indicated for the treatment of patients with paroxysmal atrial fibrillation (AF), features the POLARx FIT cryoablation balloon catheter, enabling...
Patient-tailored catheter ablation results in a significant reduction in atrial tachyarrhythmia (ATA) burden in shock-resistant persistent atrial fibrillation (AF) patients using implantable cardiac monitors (ICMs) implanted two months pre-procedure and conventional analysis may not capture the true impact of...
Ilika and Cirtec Medical have announced that they have signed a 10-year manufacturing licence to produce the Stereax range of mm-scale batteries at Cirtec’s facility in Lowell, USA.
Ilika, which is based in Romsey, UK, develops solid-state batteries, which can...
Biosense Webster has announced that several products in its cardiac ablation portfolio have received approval for a zero fluoroscopy workflow from the US Food and Drug Administration (FDA).
The products that can be used in this workflow include: Thermocool Smarttouch...
Researchers have presented a new method for assessing atrial remodelling in patients with atrial fibrillation (AF), based on the simultaneous assessment of electrical and contractile activity in the atria.
Details of the method have been set out in a paper...
The World Health Organization (WHO) has included a cardiovascular polypill—an all-in-one pill containing an antiplatelet, lipid lowering medication, and a blood pressure lowering and vascular stabilising drug (acetylsalicylic acid, ramipril, and atorvastatin)—in its List of Essential Medicines.
Developed by the...
Applying artificial intelligence (AI) to a single apical four chamber (A4C) view echocardiogram provides accurate information to detect heart failure with preserved ejection fraction (HFpEF), according to research published in JACC Advances.
The study, presented at the American Society of...
The combination of soaring heat and fine particulate pollution may double the risk of myocardial infarction (MI) mortality, according to a new study of more than 202,000 heart attack deaths in China. Findings of the study were published in...
This advertorial has been sponsored by GE Healthcare.
In this white paper Usman Siddiqui (University of Central Florida, Hunters Creek, USA) highlights reasons why the Prucka 3 with CardioLab digital recording system (GE Healthcare) stands out as the gold standard...
This post is sponsored by GE Healthcare
In this white paper, Usman Siddiqui (University of Central Florida, Hunters Creek, USA) highlight reasons why the Prucka 3 with CardioLab digital recording system (GE Healthcare) stands out as the gold standard in...
Biosense Webster has today announced the US launch of the Optrell mapping catheter with Trueref technology powered by the Carto 3 system.
The Optrell mapping catheter is a high-density diagnostic catheter, with small electrodes arranged in a fixed array formation...
Entries are now open for the inaugural edition of the Global Cardiovascular Awards, a recognition scheme highlighting the important contributions made by healthcare professionals and industry to improve outcomes for cardiovascular disease patients.
Held in association with Cardiovascular News, the...
Biosense Webster has announced that enrolment has been completed in the SmartfIRE study evaluating the safety and efficacy of its investigational Thermocool Smarttouch SF dual energy catheter and investigational Trupulse generator for the treatment of drug refractory symptomatic paroxysmal...
The use of embedded electrocardiogram (ECG) sensors in shopping trolley handles could effectively identify individuals with previously undiagnosed atrial fibrillation (AF). This is among the findings from the SHOPS-AF study, presented at the annual congress of the Association of...
The Heart Rhythm Society (HRS) has announced the appointment of Sami Viskin (Tel Aviv University, Tel Aviv, Israel) as the new editor-in-chief of Heart Rhythm from January 2024.
Heart Rhythm is the official journal of the HRS, the Cardiac Electrophysiology...
Medtronic is recalling all implantable cardioverter defibrillator (ICD) and cardiac resynchronisation (CRT-D) devices manufactured after 2017 with a glassed feedthrough, as there is a risk that the devices may deliver low or no energy output when high voltage therapy...
Stereotaxis has announced that the first patients have been successfully treated utilising Abbott’s EnSite X EP system with Stereotaxis’ robotic magnetic navigation system.
The combination of Abbott’s leading cardiac mapping system with Stereotaxis’ advanced robotic technology brings together highly detailed...
CathVision today announced its most recent financing round of US$9 million from investors.
The financing will help advance commercial operations driving adoption of the ECGenius system, the company's electrophysiology recording technology, and support the continued development of artificial intelligence (AI)-powered...
AccurKardia has announced that its flagship product, the AccurECG analysis system has been granted US Food and Drug Administration (FDA) 510(k) clearance.
AccurECG is a cloud-based, device-agnostic and fully automated electrocardiogram (ECG) interpretation software platform. The software includes beat-by-beat analysis,...
FIRE1 has announced that the first US patients have been successfully implanted with its FIRE1 system for remote heart failure monitoring in an early feasibility study (EFS).
The study will assess FIRE1’s novel solution to improve outcomes for heart failure...
Append Medical has raised US$4.35 million as part of an extended series A round, which will be used to support the company's first-in-human trials of Appligator.
Investors include participants from the first tranche of the series A round, as well as new investors,...
Sequana Medical NV has announced that the first patient has been enrolled in the MOJAVE study, a randomised controlled Phase 1/2a study in the USA, evaluating the safety and efficacy of the Company’s second-generation in diuretic-resistant DSR product (DSR...
Biotronik has announced US Food and Drug Administration (FDA) approval of its portfolio of Amvia Edge pacemakers and cardiac resynchronisation therapy pacemaker (CRT-P).
In a press release, Biotronik describes Amvia Edge as the market's smallest single-chamber MR conditional pacemaker and...
Researchers have developed a soft, fully bioresorbable, transparent microelectrode array (MEA) for monitoring heart disease and dysfunction.
Outlining the design, fabrication, characterisation and validation of the device in a paper published today in Science Advances, Zhiyuan Chen (The George Washington...
Abbott has announced that the US Food and Drug Administration (FDA) has approved the Aveir dual chamber (DR) leadless pacemaker system, described by the company as the world's first dual chamber leadless pacing system for the treatment of abnormal...
Patients who feel low when having a cardiac device implanted are more likely to stop taking their heart medications than those without depression, according to research presented at the annual congress of the Association of Cardiovascular Nursing and Allied...
Anumana has received breakthrough device designation from the US Food and Drug Administration (FDA) for its electrocardiogram (ECG) artificial intelligence (AI) algorithm designed to aid the early identification of cardiac amyloidosis.
The breakthrough device designation was granted to provide patients...
Philips, a global leader in health technology, has announced it has teamed up with Biotronik, a leading global medical device company with products and services that improve the lives of patients suffering from cardiovascular and endovascular diseases, to expand...
BioCardia, a developer of cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary diseases, has announced that the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research (CBER) has completed review of the CardiAMP...
Findings of a retrospective cohort study assessing the long-term real-world safety and efficacy of leadless pacemakers implanted in patients throughout the Netherlands are “promising for longer-term data on leadless pacing”, the study’s authors suggest.
Writing in Heart Rhythm, Karel Breeman...
Procedure-related complications and mortality rates associated with catheter ablation of atrial fibrillation (AF) are low and have declined in the past decade, conclude the authors of a systematic review and pooled analysis, published in the Journal of the American...
CorWave has raised €61 million to fund its first industrial deployment and entry into clinical trials.
SPI fund, managed by Bpifrance on behalf of the French Government as part of France 2030, and leading family controlled entrepreneurial groups were the...
Nearly one-third of patients with an implanted device to prevent sudden death have anxiety in the first year while depression affects one in five, findings of a study in EP Europace.
“Implantable cardioverter defibrillators (ICDs) are effective at extending patients’...
The European Society of Cardiology (ESC) has announced the appointment of Jean-François Riffaud as its new chief executive officer. Riffaud will replace Isabel Bardinet, who will retire after 18 years at the ESC.
Riffaud is currently CEO of the French...
Arrhythmia Alliance has announced the launch of the first SVT Awareness Day (7 June), taking place during World Heart Rhythm Week (5‒11 June) to raise awareness of what it describes as a “little-known heart rhythm disorder that affects millions”.
Supraventricular...
The US Food and Drug Administration (FDA) has released draft guidance with updated recommendations for good clinical practices (GCPs) aimed at modernising the design and conduct of clinical trials.
In a statement, the regulator said that the updates are intended...
Researchers have developed an artificial intelligence (AI) model for electrocardiogram (ECG) analysis that allows for the interpretation of ECGs as language.
This approach can enhance the accuracy and effectiveness of ECG-related diagnoses, especially for cardiac conditions where limited data is...
Defibrillators are being used in just one in 10 cardiac arrests where they are available, according to research presented at the British Cardiovascular Society Conference (5–7 June, Manchester, UK).
The research drew upon data from the East of England Ambulance...
Conformal Medical has announced the successful completion of its series D funding round, raising a total of $35 million. The company is developing the CLAAS system, which it describes as a next-generation left atrial appendage occlusion (LAAO) technology
“SPRIG Equity...
Medtronic has announced that Ken Washington has been appointed chief technology and innovation officer.
Washington joins Medtronic from Amazon where he served as vice president and general manager of consumer robotics, and will lead technology development across industries including robotics,...
Kardium has announced today that the first US patients have been successfully treated in the PULSAR clinical study, using its Globe pulsed-field ablation system.
The PULSAR study will enrol over 400 patients for treatment at up to 35 sites in...
This article was sponsored by Biosense Webster
According to data published by Patrick M McCarthy (Feinberg School of Medicine, Chicago, USA) and colleagues in the Journal of Clinical Medicine, AF affects 1%—roughly 33 million—of patients in the western population, a...
New data presented from an investigator-sponsored European trial found managing indicated heart failure patients with Abbott’s CardioMEMS HF system resulted in a significant improvement in patient-reported quality-of-life scores as early as three months after use with the remote monitoring...
Medtronic has announced US Food and Drug Administration market clearance to apply the AccuRhythm AI algorithms to the Reveal LINQ insertable cardiac monitor (ICM), the predecessor to the LINQ II ICM, via cloud-based updates.
Medtronic anticipates the AccuRhythm AI algorithms...
An international clinical trial presented today at the European Stroke Organisation Conference (ESOC; 24–26 May, Munich, Germany) has demonstrated that—in people with ischaemic stroke and atrial fibrillation—anticoagulation can safely be started earlier than current guidelines recommend.
"Our study finally brings...
Preliminary results from the BIO-LIBRA study, assessing the outcomes of device-treated ventricular arrhythmias or mortality in patients with non-ischaemic cardiomyopathy (NICM) being treated with implantable cardioverter-defibrillators (ICDs) or cardiac resynchronisation therapy defibrillators (CRT-Ds) along with guideline-directed medical therapy (GDMT)...
Findings from a new clinical trial support use of implantable cardiac monitors (ICM) as a standard of care in managing patients with complex cardiac arrhythmias like atrial fibrillation (AF). The study will be presented today as a late-breaking clinical...
Results from a pivotal clinical trial found a leadless pacemaker can deliver cardiac resynchronisation therapy (CRT) among patients who were not able to be treated with conventional CRT and epicardial leads.
The novel WiSE CRT system removes the possibility of...
Abbott has announced that the US Food and Drug Administration (FDA) has approved the company's TactiFlex Ablation Catheter, Sensor Enabled, which features a flexible tip and contact force technology.
Used to perform an ablation procedure to treat atrial fibrillation (AF),...
A novel artificial intelligence (AI) model correctly identified patients at near-term risk of sustained ventricular tachycardia (VT) who could potentially benefit from preemptive interventions to prevent sudden cardiac death (SCD).
The AI-model utilises a single-lead electrocardiogram (ECG) screening tool that...
Results from a clinical trial, presented at the 2023 Heart Rhythm Society annual meeting (May 19–21, New Orleans, USA), indicate that overweight and obese patients with persistent and paroxysmal atrial fibrillation (AF) who lose weight prior to a catheter...
New data from three late-breaking clinical trials has demonstrated the safety and efficacy of pulsed field ablation (PFA) as a viable, non-thermal treatment option for atrial fibrillation (AF) and were presented at the 2023 Heart Rhythm (May 19–21, New...
Medtronic has announced findings from a secondary analysis of the PULSED AF study, demonstrating positive results for the Medtronic PulseSelect pulsed field ablation (PFA) system including atrial arrhythmia (AA) burden reduction, which correlated to improved quality of life and...
Stereotaxis has announced a global collaboration with Abbott to integrate Abbott’s EnSite X EP system with Stereotaxis’ Robotic Magnetic Navigation system.
The combination of Abbott’s cardiac mapping system with Stereotaxis’ advanced robotic technology brings together highly detailed real-time diagnostic information...
Late-breaking results from the AVEIR dual-chamber (DR) i2i Investigational Device Exemption (IDE) study, a large-scale study to assess Abbott’s Aveir dual-chamber leadless pacemaker, have been presented at the Heart Rhythm Society’s 2023 annual meeting (19–21 May, New Orleans, USA)...
Impulse Dynamics has today announced the completion of the first implantation for the INTEGRA-D clinical trial, designed to evaluate the safety and efficacy of two proven cardiac therapies combined—cardiac contractility modulation (CCM) and an implantable cardioverter defibrillator (ICD)—in a single...
MicroPort, a global developer in the field of cardiac rhythm management, announced today it has received US Food and Drug Administration (FDA) approval for its latest range of implantable pacemakers, Alizea and Celea, the longest-lasting pacemakers for their size on...
Biosense Webster—a stem of Johnson & Johnson MedTech—announced that data from the Q-FFICIENCY study was published in JACC: Clinical Electrophysiology. The study evaluated the safety and 12-month effectiveness of the QDot Micro catheter in paroxysmal atrial fibrillation (AF) ablation...
A recent study has found deep neural networks (DDNs)—a category of artificial intelligence (AI) algorithm—capable of forecasting cardiac pump function from standard angiography videos, providing accurate left ventricular ejection fraction (LVEF) predictions with strong correlations to echocardiographic LVEF measurements,...
AstraZeneca’s Farxiga (dapagliflozin) has been approved in the USA to reduce the risk of cardiovascular (CV) death, hospitalisation for heart failure (hHF) and urgent heart failure (HF) visits in adults with HF.
The approval by the Food and Drug Administration...
Biotronik announced today the latest addition to its cardiac rhythm management portfolio.
"We are excited to have received CE mark for our newest technology—the world’s first pacemakers and CRT-Ps approved for left bundle branch pacing. Amvia Sky and Amvia Edge...
Adagio Medical has announced the completion of enrolment in its Cryocure-VT trialof ultra-low temperature cryoablation for the treatment of monomorphic ventricular tachycardias.
Sixty patients with ischaemic and non-ischaemic cardiomyopathies underwent an endocardial ultra-low temperature cryoablation procedure using Adagio's vCLAS catheter...
Medtronic has received US Food and Drug Administration (FDA) approval of its Micra AV2 and Micra VR2 leadless pacemakers.
Micra AV2 and Micra VR2, the world's smallest pacemakers, provide longer battery life and easier programming than prior Micra pacemakers, while...
CathVision, a medical technology company developing innovative electrophysiology solutions to enhance clinical decision-making in the electrophysiology (EP) lab, today announced initial patient enrolment of a follow-on clinical study to demonstrate the value of the Signal Complexity algorithm led by...
Results from the MATRIX study show that the high detection accuracy of DX single-lead implantable cardioverter defibrillator (ICD) systems for atrial fibrillation (AF) episodes (99.7% for ≥1h episodes) in combination with the strong transmission performance of Biotronik home monitoring...
Effective management of depression through psychological therapy is associated with a lower likelihood of heart disease and stroke, according to research published today in European Heart Journal, a journal of the European Society of Cardiology (ESC).
“Our study suggests that...
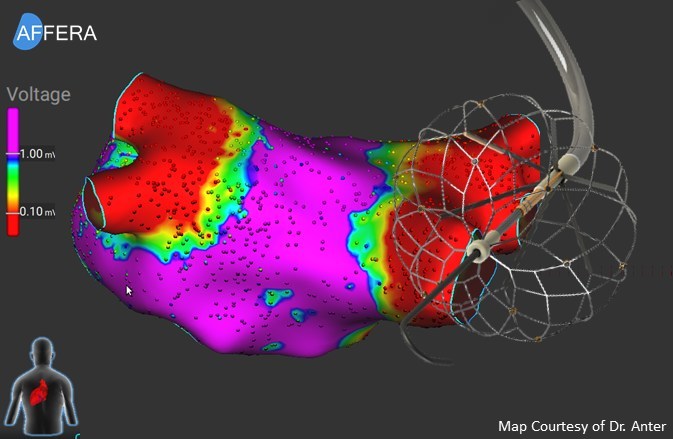
Medtronic have announced the 12-month findings that supported the Affera Mapping and Ablation system CE Mark, demonstrating that the Sphere-9 catheter can successfully treat patients with either paroxysmal or persistent atrial fibrillation (AF) using a variety of ablation lesion...
Patients with severe COVID-19 requiring mechanical ventilation are 16 times more likely to develop ventricular tachycardia within six months compared to their peers without severe infection, according to research presented at the annual congress of the European Heart Rhythm...
A score based on four readily available clinical and imaging parameters identifies the heart failure patients who benefit most from atrial fibrillation (AF) ablation, according to late breaking science presented at the annual congress of the European Heart Rhythm Association...
An innovative three-step ablation approach including ethanol infusion of the vein of Marshall improves freedom from arrhythmias in patients with persistent atrial fibrillation (AF) compared to pulmonary vein isolation (PVI) alone, according to late breaking science presented at the...
A novel software tool set to improve the management of elderly atrial fibrillation (AF) patients with multiple conditions is being designed by the EU-funded and coordinated by the European Society for Cardiology (ESC) European Heart Rhythm Association (EHRA)-PATHS consortium....
High power electric vehicle chargers are safe for patients with pacemakers and defibrillators, according to a study published in EP Europace, a journal of the European Society of Cardiology (ESC) and presented at the annual congress of the European...
Spanish footballer, Iker Casillas, was awarded an European Heart Rhythm Association (EHRA) Gold Medal for services to cardiovascular health yesterday at the 2023 annual meeting.
In 2019, Iker, captain of the Spanish 2010 World Cup-winning team, suffered a heart attack...
Thirty-day electrocardiogram (ECG) monitoring in patients with hypertrophic cardiomyopathy (HCM) detects more arrhythmias than the standard 24 to 48 hours, according to late breaking science presented at the annual congress of the European Heart Rhythm Association (EHRA 2023, 16–18...
A region-wide study in more than 50,000 patients with atrial fibrillation (AF) has found reduced risks of stroke and transient ischaemic attack in those who started statins within a year of diagnosis compared with those who did not. The...
Stereotaxis has announced that over 1,000 cardiac arrhythmia patients have been treated using the Genesis Robotic Magnetic Navigation (RMN) system.
Genesis RMN, was first used in late 2020 following regulatory clearances in the USA and Europe. Since then, physicians at...
Boston Scientific has received CE mark for its POLARx FIT cryoballoon catheter for atrial fibrillation (AF) ablation.
According to the company, the device is the only cryoablation system that offers a dual diameter balloon size in one catheter to deliver an individualised...
Daytime napping for 30 minutes or longer is associated with an increased likelihood of developing atrial fibrillation (AF), according to research presented at European Society of Cardiology (ESC) Preventive Cardiology 2023, a scientific congress of the ESC.
“Our study indicates...
Wireless or leadless pacemakers, commonly implanted in adults, may be a safe and effective short-term option for children with slow heartbeats, according to new research published today in Circulation: Arrhythmia and Electrophysiology, a peer-reviewed journal of the American Heart...
SentiAR has closed a Series B financing round worth US$8.5 million. The financing was led by cultivate (MD) Ventures and joined by MedVenture Partners alongside several insider investors, including TechWald Holding, VCapital, QRM Capital, and Harmonix Fund. The funding...
Medtronic has announced the launch of MRI Care Pathway, a new system designed to streamline the process of completing magnetic resonance imaging (MRI) scans for patients with Medtronic MRI-compatible cardiac devices.
According to a company press release, research shows that...
Endotronix has announced its PROACTIVE-HF pivotal study has successfully completed enrolment.
The study is designed to evaluate the safety and efficacy of the Cordella pulmonary artery (PA) sensor, and the resulting data will support the premarket approval (PMA) application for...
Wearable devices such as smart watches could be used to detect a higher risk of developing heart failure and irregular heart rhythms in later life, suggests a new study led by University College London (UCL) researchers.
The peer-reviewed study,...
The Society for Cardiovascular Angiography & Interventions (SCAI) and the Heart Rhythm Society (HRS) have released an updated expert consensus statement on transcatheter left atrial appendage closure (LAAC).
In a press release, SCAI and HRS say that they prioritised the...
Results from the PADN-5 study, investigating the efficacy and safety of pulmonary artery denervation in the treatment of combined postcapillary and precapillary and pulmonary hypertension (CpcPH) related to left heart failure, have been presented at the Technology and Heart...
More than 40% of women report anxiety four months after a cardiac arrest compared with 23% of men, according to research presented at the scientific congress of the European Society of Cardiology (ESC) Acute CardioVascular Care 2023 (24–26 March,...
Patients with atrial fibrillation (AF) have been said to benefit from early rhythm control therapy, which reduces cardiovascular deaths, strokes, and other adverse outcomes by 20% compared to usual care, the European Society of Cardiology (ESC) has stated following...
Procyrion, a medical device company which aims to improve outcomes for patients with cardiac and renal impairment, announced today that use of its Aortix percutaneous mechanical circulatory support (pMCS) device led to rapid decongestion in a pilot study of...
Abbott has today announced new data that found monitoring patients remotely with haemodynamic pressure sensing technology, such as with its CardioMEMS HF System, can significantly improve survival in heart failure patients with reduced ejection fraction (HFrEF). The analysis is...
Vektor Medical has announced that use of the vMap arrhythmia mapping system during complex atrial fibrillation (AF) ablation was associated with significantly improved freedom from atrial arrhythmias compared with standard-of-care pulmonary vein isolation. Results will be presented at the...
Stereotaxis has announced the opening of a new robotic electrophysiology programme at Broward Health Medical Center in Fort Lauderdale, USA. Broward Health is among the first in the nation, and the first in Florida, to offer the latest Genesis...
A total of £10 million has been awarded to the Medicines and Healthcare products Regulatory Agency (MHRA)—an executive agency of the UK Department of Health and Social Care (DHSC)—to help bring innovative new medicines and medical technologies to UK...
HeartBeam, a cardiac technology company that has developed the three-dimensional (3D)-vector electrocardiogram (VECG) platform for heart attack detection today announced the strategic acquisition of all assets from LIVMOR, a digital health solutions company providing a patient-engaging remote monitoring system...
Kardium, a private medical device company that has developed the Globe pulsed field (PF) system for the treatment of atrial fibrillation (AF) using pulsed field ablation (PFA), announced today that the first patients have been successfully treated in its...
Acesion, the biotech developing first-in-class novel therapies for atrial fibrillation (AF), has today announced positive data from its phase two trial of AP30663, a first-in-class SK ion channel inhibitor for conversion of AF to normal sinus rhythm.
The trial enrolled...
Medtronic has announced today that it has received CE Mark for the Affera mapping and ablation system, which includes the Sphere-9 catheter and the Affera Prism-1 mapping software.
The full system creates a new paradigm in electrophysiology through the...
A recent analysis of a large nationwide database of patients with atrial fibrillation (AF) who underwent ablation has reported an independent association between being underweight and an increased risk of cardiac tamponade during ablation.
Cardiac tamponade is a potentially...
A retrospective cohort study investigating the rate of maternal characteristics associated with, and survival following, cardiac arrest during delivery hospitalisation, has found that older, non-Hispanic Black and low-income pregnant patients are disproportionately affected, but maintain better survival rates.
Published...
Using data from a large and “nationally representative” sample of the US population, a recent study is the first—the authors posit—to demonstrate racial differences in in-hospital outcomes after atrial fibrillation (AF) ablation in patients with heart failure (HF).
Published...
A recent study has provided new mechanistic insights identifying distinct atrial electrophysiological remodelling and fibrosis-associated conduction abnormalities favouring atrial fibrillation (AF) susceptibility in heart failure with preserved ejection fraction (HFpEF). The results from this proof-of-concept study support a correlation...
Biosense Webster has announced the first cases with its investigational Thermocool Smarttouch SF dual energy catheter have taken place as part of the SmartfIRE Study.
The first procedure was performed by Tom De Potter at OLV Hospital in Aalst, Belgium....
The European Union’s Council of Ministers has today adopted a resolution to extend the deadline for the certification of medical devices under the Medical Devices Regulation (MDR).
Producers of medical devices will have until 31 December 2027 for higher risk...
Results of the Pulsed AF Pivotal trial, investigating pulsed field ablation (PFA) treatment in patients with paroxysmal and persistent atrial fibrillation (AF), have been shared at the American College of Cardiology (ACC) 2023 Scientific Sessions (4 – 6 March,...
UC San Francisco (UCSF) is conducting a six-month clinical trial on hypertrophic cardiomyopathy (HCM) using Vivalink's Biometrics Data Platform. The study, consisting of 70 patients, will evaluate if regimented moderate intensity exercise improves overall exercise capacity and cardiac blood...
Early rhythm control one year after atrial fibrillation (AF) diagnosis has been found to be beneficial in preventing recurrent stroke in patients with incident AF and a prior history of stroke. Published in JACC: Clinical Electrophysiology, researchers suggest that...
Massachusetts Institute of Technology engineers have created a soft robotics-enabled three-dimensional (3D)-printed anatomical hydrodynamic system that can recreate the hemodynamic system of aortic stenosis (AS) and congenital defects in specific patient cases.
The researchers first took medical images of...
Biotronik has received CE mark approval for the Selectra 3D implant tools to include left bundle branch area pacing (LBBAP) in addition to His-bundle pacing (HBP).
Commonly referred to as conduction system pacing (CSP) these two approaches have emerged as...
Disabilities were underreported in clinical trial data and commonly used as a basis for exclusion from trial participation in an analysis of 80 recent trials involving cardiovascular outcomes, according to a study being presented at the American College of...
Atrial fibrillation (AF) ablation carried out in an inpatient setting is associated with higher rates of early mortality and subsequent complications when compared with outpatient AF ablation, a new study finds.
Referring to the Medicare fee-for-service (FFS) database, Mary-Jo...
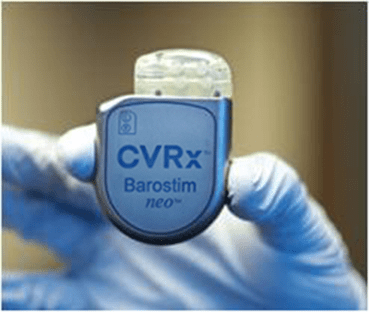
CVRx has announced the preliminary topline results of the BeAT-HF—Baroreflex activation therapy for heart failure—postmarket randomised clinical trial.
The BeAT-HF postmarket phase of the multicentre, prospective, randomised controlled trial assessed 323 patients suffering from heart failure with reduced ejection fraction....
A new “red flag-raising” study has provided benchmark data on the safety of smart scales, watches and rings with bioimpedance technology for patients with cardiovascular implantable electronic devices (CIEDs). Discussing the research, principal investigator Benjamin Sanchez Terrones (University of...
Brainomix has announced its involvement in a new study sponsored by the University of Liverpool (Liverpool, UK) focused on post-stroke atrial fibrillation (AF). Sites with existing clinical deployments of Brainomix's e-Stroke platform will utilise the artificial intelligence (AI) system...
Medtronic has received CE mark for the Aurora extravascular implantable cardioverter-defibrillator (EV-ICD) magnetic resonance imaging (MRI) SureScan device and Epsila EV MRI SureScan defibrillation lead to treat dangerously fast heart rhythms that can lead to sudden cardiac arrest (SCA)....
A retrospective analysis of randomised clinical trials has found that one in five patients aged 70 years or older with cardiovascular risk factors are diagnosed with bradyarrhythmias when long-term monitoring for atrial fibrillation (AF) using implantable loop recorder (ILR)...
Kareem Abdul-Jabbar is working with Bristol Myers Squibb and Pfizer to raise awareness of atrial fibrillation (AF) and its symptoms as part of the No Time to Wait campaign.
In support of the campaign, Abdul-Jabbar will share his experience with...
University of Texas (UT) Southwestern Medical Center researchers have mapped gene control elements in specialised cardiac cells responsible for heartbeat regulation. The findings of the genome exploration study, published in the Journal of Clinical Investigation, provide insight into how...
Acutus Medical has announced results from the AcQForce Flutter study, evaluating use of the AcQBlate Force sensing ablation catheter and system in the treatment of right atrial typical flutter. Results were presented during a late-breaking clinical trials and first...
GE HealthCare has signed an agreement to acquire Caption Health, a developer of artificial intelligence (AI)-driven ultrasound technology.
The Caption Health technology—Caption AI—has been developed to detect signs of diseases such as heart failure in at-risk patients, potentially preventing hospitalisations...
In a study presented yesterday at the International Stroke Conference (ISC; 8–10 February, Dallas, USA), irregular heart rhythms were detected in roughly one in five people who survived an ischaemic stroke due to atherosclerosis and were continuously monitored for...
Biosense Webster has announced an update and late-breaking data from trials across radiofrequency ablation (RFA) and pulsed field ablation (PFA) at the 2023 AF Symposium (2–4 February, Boston, USA).
An analysis of the inspIRE-sponsored clinical trial evaluating the safety...
Abbott has today announced receipt of the CE mark for the TactiFlex ablation catheter, sensor enabled (SE), alongside US Food and Drug Administration (FDA) approval for an expanded indication of its FlexAbility ablation catheter, sensor enabled (SE).
In a press...
Among patients with heart failure with preserved ejection fraction (HFpEF) and a pacemaker, treatment with a moderately accelerated, personalised pacing rate resulted in an improved quality of life, N-terminal pro–brain natriuretic peptide (NT-proBNP) levels, and atrial fibrillation (AF) compared...
AtriCure has announced that the first patient was treated in the LeAAPS clinical trial, a prospective, randomised clinical trial to evaluate the safety and effectiveness of the AtriClip (Atricure) left atrial appendage (LAA) exclusion system for the prevention of...
Conformal Medical has announced the presentation of one-year data from the company’s initial left atrial appendage occlusion (LAAO) cases with its novel CLAAS system in patients under conscious sedation without general anaesthesia.
Vivek Reddy, (Mount Sinai Hospital, New York, USA)...
Researchers have found a correlation between periodontitis and fibrosis scarring to an appendage of the heart’s left atrium that can lead to atrial fibrillation (AF) in a sample of 76 patients with cardiac disease.
Periodontitis, a gum disease, can...
The prospective cohort study of 626 patients with non-magnetic resonance imaging (MRI)-conditional implantable cardioverter defibrillators (ICDs) suggests said ICDs remain suitable to treat detected tachyarrhythmias post-1.5-Tesla MRI.
Previous reports of adverse events have typically been noted during or immediately after...
BioVentrix, a privately held medical device company focused on the development of less invasive therapies to directly treat the dilated left ventricle and reverse the left ventricular remodelling process of progressive heart failure, today announced the US Food and...
tenacio, a company focused on digitising cardiovascular care to improve patient outcomes and reduce healthcare costs, has announced its OptiCor patient management platform is now commercially available following a completed review by the US Food and Drug Administration (FDA).
At...
Varian, associated with Siemens Healthineers, announced today that the US Food and Drug Administration (FDA) approved an investigational device exemption (IDE) for the pivotal RADIATE-VT clinical trial. The trial will be the first international, multi-centre, randomised controlled trial to...
An international study conducted by Leonard Yeo (National University Hospital, Singapore) and colleagues has found that the presence of atrial fibrillation does not modify the treatment effect of bridging intravenous thrombolysis (IVT) in ischaemic stroke patients undergoing a thrombectomy...
Ra Medical Systems announces the completion of its previously-announced stock-for-stock merger transaction with Catheter Precision, a company focused on the cardiac electrophysiology market.
MedTech veteran and Catheter Precision CEO and founder David Jenkins has joined Ra Medical’s board of directors...
Biobeat, a global leader in wearable remote patient monitoring solutions for the healthcare continuum, announced today that its wearable remote patient monitoring devices have received 510(k) clearance from the US Food and Drug Administration (FDA) to monitor stroke volume...
CardiacSense, a digital health company that developed the world's most advanced, medically certified wearable device for monitoring vital signs, announced receipt of US Food and Drug Administration (FDA) clearance of its CSF-3 watch for measuring beat-by-beat heart rate, and...
In their updated meta-analysis of 4,175 patients, Stergios Intzes together with a team from the Democritus University of Thrace, Alexandroupoli, Greece and the Heart Center, University of Leipzig, Germany, found P-wave duration (PWD) to be an independent predictor of...
HeartBeam, a cardiac technology company that has developed a 3D-vector electrocardiogram (ECG) platform for heart attack detection, has announced that a patent that enables generation of a synthesised 12-lead ECG by the HeartBeam AIMIGo credit card-sized device was issued...
A preclinical study demonstrating key features of the foam-based left atrial appendage closure device (CLAAS, Conformal Medical) has been published in the Journal of the Society for Cardiovascular Angiography and Interventions (JSCAI).
“This study validates the design features of Conformal's...
Cardiac Insight, a specialist in prescription-based wearable cardiac sensors and automated electrocardiogram (ECG) analysis software for cardiac arrhythmia diagnosis, has announced that researchers at Stanford Medicine selected the company’s Cardea SOLO wearable ECG system for a medical study entitled:...
Volta Medical has announced €36 million in new series B funding, bringing the total capital raised to over €70 million.
The company is developing artificial intelligence (AI) solutions to assist electrophysiologists in treating complex cardiac arrhythmias such as atrial fibrillation...
Anthos Therapeutics has announced that it has enrolled the first patient in LILAC-TIMI 76, a phase 3 study to evaluate the efficacy and safety of abelacimab in high-risk patients with atrial fibrillation (AF) deemed unsuitable for current anticoagulants by...
Johnson & Johnson has today announced it has completed its acquisition of Abiomed. Abiomed is now part of Johnson & Johnson and will operate as a standalone business within Johnson & Johnson’s MedTech segment.
“We are excited to officially welcome...
Primary care visits rise sharply in the weeks immediately preceding a sudden cardiac arrest, according to results from the ESCAPE-NET project. The project is backed by the European Heart Rhythm Association (EHRA) of the European Society of Cardiology (ESC)...
A cohort study of more than 4,500 persons without a history of atrial fibrillation (AF) or stroke has found that measuring left atrial mechanical function can improve stroke prediction. The findings are published in Annals of Internal Medicine.
AF is a...
The current model for randomised clinical trials must be redesigned for the modern age, according to the European Society of Cardiology (ESC), American Heart Association (AHA), World Heart Federation (WHF) and American College of Cardiology (ACC).
The call comes in...
Field Medical and CardioNXT have announced a strategic collaboration to provide the first-of-its-kind focal pulsed field ablation (PFA) therapy integrated with 3D mapping & navigation.
"PFA has generated much excitement in the treatment of atrial fibrillation due to an improved...
Biotronik announce the first patient enrolment in BIO-CONDUCT, an US Food and Drug Administration (FDA) approved Investigational Device Exemption (IDE) trial examining the use of the Biotronik Solia S pacing lead when implanted in the left bundle branch (LBB)...
With numerical simulations, researchers have demonstrated a new way to time weak electrical pulses that can stop certain life-threatening arrhythmias. Publishing their work in Chaos, by AIP Publishing, the group shows that timed pulses are successful in ending atrial...
Based in the USA, Weill Cornell Medicine has initiated a new clinical project together with the Mwanza International Trials Unit located in the Lake Zone of Tanzania with the aim of exploring the burden of cardiac arrythmias in adults...
Europe’s health commissioner, Stella Kyriakides, has announced that proposals to extend the transition period for the implementation of the European Union’s (EU) Medical Device Regulation (MDR) will be put forward in early 2023.
Kyriakides informed health ministers from the EU’s...
AstraZeneca has today announced that the Medicines and Healthcare products Regulatory Agency (MHRA) has granted a licence extension for dapagliflozin (Forxiga) in Great Britain for the treatment of symptomatic chronic heart failure (HF) in patients with HF and a...
The European Society of Cardiology (ESC) has urged European Union (EU) health ministers to act to prevent a “shortfall of essential medical devices for cardiovascular patients”, caused by challenges in the implementation of Europe’s Medical Device Regulation (MDR). Diagnostic...
Ultromics’ EchoGo Heart Failure, an artificial intelligence (AI) solution for echocardiography with the potential to revolutionise the diagnosis of heart failure with preserved ejection fraction (HFpEF), has received US Food and Drug Administration (FDA) clearance.
The system was developed by...
Medtronic has announced enrolment completion and final treatment in the SPHERE Per-AF, a US Food and Drug Administration (FDA) Investigational Device Exemption (IDE) trial. The study aims to evaluate the safety and efficacy of the Sphere-9 pulsed field (PF)...
A first-of-its-kind analysis on electrophysiological findings in patients with recurrent atrial tachyarrhythmia (ATa) following pulmonary vein isolation (PVI) using the novel pentaspline pulsed-field ablation (PFA) catheter reports a low incidence of pulmonary vein (PV) reconnection.
Shonta Tohoku (Cardiovascular Centre Bethanien,...
More scientific evaluation is needed to develop and validate consensus recommendations to ensure that benefits will outweigh risks for consumers who use smartwatches in screening themselves for possible atrial fibrillation (AF), according to the authors of an article published...
The Swiss Federal Assembly has voted in favour of accepting medical devices with US Food and Drug Administration (FDA) marketing authorisation in Switzerland.
A motion for ‘more freedom of action in the procurement of medical products for supply of the...
Abbott has been recognised by the Consumer Technology Association (CTA) with three Consumer Electronics Show (CES 2023; 5–8 January, Las Vegas, USA) Innovation Awards for multiple technologies that are said to be “advancing the health tech industry and improving...
An analysis of randomised controlled trials (RCTs) has found catheter ablation reduces mortality and heart failure hospitalisations in patients with atrial fibrillation (AF) and heart failure. Findings of the study were published in the journal Europace.
Carrying out a meta-analysis...
HeartBeam, a cardiac technology company that has developed the first and only 3D-vector electrocardiogram (VECG) platform for heart attack detection has announced that its patent for a 12-lead electrocardiogram (ECG) smartwatch-based monitor intended for detection of myocardial infarction (MI)...
Abbott has announced that Health Canada has approved the Aveir single-chamber (VR) leadless pacemaker.
The Aveir leadless pacemaker is implanted directly inside the heart's right ventricle via a minimally invasive procedure to treat slower-than-normal heart rates. Unlike traditional pacemakers, leadless...
A recent presentation by Paul J Wang (Stanford School of Medicine, Stanford, USA) at the American Heart Association (AHA) Scientific Session 2022 conference (5-7 November, Chicago, USA) outlined the results of the ENHANCE-AF study into a new shared decision-making...
Biosense Webster has announced the publication of data from a study comparing the risk of dementia in patients with atrial fibrillation (AF) who were treated with catheter ablation versus anti-arrhythmic drugs (AAD) in the American Heart Journal.
The study evaluated...
CVRx—the developer of the “world’s first” US Food and Drug Administration (FDA)-approved neuromodulation device to treat the symptoms of heart failure—has launched its new Barostim NEO2 implantable pulse generator (IPG).
The second-generation device reduces the size of the IPG by...
Ablation as a first-line treatment for atrial fibrillation (AF) disease is associated with significantly better clinical outcomes than starting with antiarrhythmic drugs.
These were the findings of the 36-month PROGRESSIVE-AF trial, presented by Jason Andrade (Vancouver General Hospital, Vancouver, Canada)...
WL Gore & Associates has announced that it is initiating the RELIEF clinical study—an investigational study to evaluate the safety and efficacy of transcatheter closure of patent foramen ovale (PFO) for the relief of migraine headaches utilising the Gore...
Screening to detect atrial fibrillation (AF) in older people would not only increase the chance of preventing stroke, it would also save money for the healthcare system and society. This is the conclusion from research published in the European...
iRhythm Technologies has announced a new health economic analysis of the mSToPS study, presented at the American Heart Association (AHA) scientific sessions 2022 (5–7 November, Chicago, USA).
The study evaluated the cost-effectiveness of screening for atrial fibrillation (AF) with Zio...
CathVision has announced the investigation of the Signal Complexity algorithm designed to visualise and quantify atrial fibrillation (AF) complexity parameters in patients with persistent AF. Ten patients have been treated to date at NYU Langone Health (New York, USA).
CathVision’s...
Acutus Medical has announced that it has achieved the first milestone under the asset purchase agreement of its left-heart access portfolio with Medtronic.
This triggers a US$20 million earnout payment from Medtronic to Acutus and allows Acutus to become an...
Boston Scientific has announced the European launch of the LUX-Dx insertable cardiac monitor (ICM) system, a long-term diagnostic device inserted under the skin of patients to detect arrhythmias associated with conditions such as atrial fibrillation (AF), cryptogenic stroke and...
Kardium has announced the successful first-in-human study of its next generation Globe pulsed field system to treat atrial fibrillation (AF) using pulsed field ablation (PFA) therapy.
Working with Vivek Reddy (Mount Sinai Hospital, New York, USA), and Petr Neužil...
Use of apixaban was associated with a lower risk of gastrointestinal bleed and similar rates of ischaemic stroke or systemic embolism, intracranial haemorrhage and all-cause mortality compared to other direct oral anticoagulants in patients with atrial fibrillation (AF), research...
Johnson & Johnson is to acquire Abiomed, it was announced today, after the two organisations entered into a definitive agreement under which Johnson & Johnson will acquire all outstanding shares of Abiomed through a tender offer.
The transaction will include...
Adagio Medical has announced its first pulsed field cryoablation (PFCA) procedure performed using the Adagio CryoPulse catheter system as part of the PARALELL (Pulsed field ablation and pulsed field cryoabLation in perrsistent atriaL fibriLlation) clinical trial.
The procedure was performed...
Researchers are to investigate the safety and efficacy of performing left atrial appendage occlusion in patients at high risk for atrial fibrillation (AF) who are undergoing cardiac surgery to prevent future stroke.
Richard Whitlock (McMaster University, Ontario, Canada) outlined the...
AccurKardia, a software company that provides clinical-grade, device agnostic, automated electrocardiogram (ECG) analytics, has announced it will collaborate with Mawi, a medtech provider of medical grade wearables in the cardiac space, to integrate its proprietary ECG analytics into Mawi’s...
In a large, population-based study of patients with atrial fibrillation (AF) and valvular heart disease, those receiving apixaban had a lower risk for ischaemic stroke or systemic embolism and bleeding, and a lower rate of intracranial or gastrointestinal (GI)...
Medtronic has received US Food and Drug Administration (FDA) approval for expanded labelling for the SelectSecure Model 3830 cardiac lead for left bundle branch area pacing.
In 2018, the FDA approved the lead for His-Bundle pacing, another form of conduction...
Catheter ablation of atrial fibrillation (AF) has favourable effects on cerebral blood flow, particularly in non-paroxysmal AF, research published in JACC: Clinical Electrophysiology.
The results may partially explain the association between cognitive decline and AF, Yoshihide Takahashi (Tokyo Medical and...
Biosense Webster has announced the European launch of the Heliostar radiofrequency balloon ablation catheter. Heliostar is indicated for use in catheter-based cardiac electrophysiological mapping of the atria and, when used with a compatible multi-channel radiofrequency generator, for cardiac ablation.
The...
Vektor Medical has announced study results that demonstrate a reduction in total procedural duration, fluoroscopy use, and cost after implementation of vMap, its cardiac mapping technology that uses only 12-lead electrocardiogram (ECG) data.
The research was presented by Avinash Toomu...
A real-world study of smartwatch electrocardiography (ECG) tools for the detection of atrial fibrillation (AF) has found that the use of such devices is “challenging” in patients with abnormal ECGs, and could be aided by better algorithms and machine...
Researchers have used artificial intelligence (AI) to evaluate patients’ electrocardiograms (ECGs) in a targeted strategy to screen for atrial fibrillation (AF). In the digitally-enabled, decentralised study, AI identified new cases of AF that would not have come to clinical...
Puzzle Medical Devices was announced as the winner of the Shark Tank innovation competition at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA).
The company is developing a novel circulatory support device that is implanted percutaneously in...
This year marked the 20th edition of the Atrial Fibrillation Symposium (AFIB2022; 24–25 May, Copenhagen, Denmark) which, over its lifetime, has evolved to become one of the leading events in the calendar for atrial fibrillation (AF) specialists. Mark O’Neill...
Medtronic has announced that the Linq II insertable cardiac monitor (ICM) system has received 510(k) clearance from the US Food and Drug Administration (FDA) for use in paediatric patients over the age of two who have heart rhythm abnormalities...
Long-term follow-up of patients with non-valvular atrial fibrillation (AF) receiving the Amplatzer Amulet left atrial appendage occlusion (LAAO) device have shown continued safety and efficacy through three-years, results from the Amulet IDE trial shared at the 2022 Transcatheter Cardiovascular...
Implantable cardioverter defibrillator (ICD) information on YouTube is of low and highly-variable quality, according to research presented at the American College of Cardiology (ACC) Quality Summit 2022 (14–16 September, Los Angeles, USA).
As more patients turn to the internet for...
A study in Circulation: Arrhythmia and Electrophysiology has identified new-onset atrial fibrillation (AF) in one in 20 patients hospitalised with COVID-19.
Using data from the American Heart Association’s (AHA’s) COVID-19 Cardiovascular Disease Registry, researchers examined nearly 28,000 patients without a...
A new report from the American Heart Association’s (AHA’s) National Cardiac Implantable Electronic Device (CIED) Infection Initiative aims to improve adherence to guidelines on device explant following an infection.
The report is the result of a summit of key opinion...
Ra Medical Systems has entered into a merger agreement with Catheter Precision.
Under the terms of the agreement, Catheter Precision will become a wholly owned subsidiary of Ra Medical in a stock-for-stock reverse merger transaction.
If completed, the merger will result...
Haemonetics Corporation has received CE mark certification for its Vascade vascular closure and Vascade MVP venous vascular closure systems.
The CE marking will allow Haemonetics to engage in the next steps of country-specific entrance of both products into the...
Results of an analysis of Aktiia’s cuffless blood pressure (BP) monitoring system are being presented at the American Heart Association (AHA) Hypertension Scientific Sessions 2022 (7–10 September, San Diego, USA).
The analysis has been co-authored by experts from Barts NIHR...
Biotronik has announced a partnership with AliveCor, the developer of personal electrocardiogram (ECG) technology and services.
The partnership will bring together Biotronik’s Biomonitor injectable cardiac monitor with AliveCor's AI-enabled, clinically validated, medical-grade KardiaMobile 6L and KardiaMobile Card ECG technology.
Through this...
The US Food and Drug Administration (FDA) has granted an investigational device exemption (IDE) to Attune Medical to evaluate the company’s ensoETM in the reduction of oesophageal thermal injury during cardiac radiofrequency (RF) ablation procedures.
The study—Improving oesophageal protection during...
Vektor Medical has announced the publication of a peer-reviewed manuscript of the clinical data from a study evaluating the accuracy of its vMap technology in Circulation: Arrhythmia and Electrophysiology.
The system, which was cleared by the US Food and Drug...
AtriAN Medical has announced that it has completed enrolment of a second study using its selective pulsed field ablation (PFA) technology for the treatment of atrial fibrillation (AF).
The Neural AF-2 study has enrolled cardiothoracic surgery patients with paroxysmal AF,...
Biosense Webster has announced the release of the Octaray mapping catheter, developed for the mapping of cardiac arrhythmias including atrial fibrillation (AF). The catheter comes with TRUEref technology— a novel mapping reference electrode—powered by the Carto 3 version 7...
Boston Scientific has received US Food and Drug Administration (FDA) approval to expand the instructions for use labelling for the current-generation Watchman FLX left atrial appendage closure (LAAC) device to include a 45-day dual anti-platelet therapy (DAPT) option as...
Data from the PROMET and RELEASE studies provide important new insights into the safety and efficacy of rotational transvenous lead extraction (TLE), Christoph Starck (Berlin, Germany) and Peter Paul Delnoy (Zwolle, The Netherlands) tell Cardiac Rhythm News at the 2022 European...
LifeTech has announced that a US Food and Drug Administration (FDA)-approved investigator-initiated pre-market clinical trial of its proprietary LAmbre Plus left atrial appendage (LAA) closure system has obtained medical insurance coverage in the USA.
This is expected to facilitate the...
The addition of posterior wall isolation (PWI) does not improve arrhythmia outcomes compared with pulmonary vein isolation (PVI) alone in patients undergoing first time ablation for persistent atrial fibrillation (AF).
This is the concluding finding from the randomised, multicentre CAPLA...
Volta Medical has announced that peer-reviewed results from the company’s Ev-AIFib proof of concept study with its VX1 decision support software has been published in the Journal of Cardiovascular Electrophysiology (JCE).
The artificial intelligence (AI) software is designed to assist...
Researchers have developed a risk score to predict mortality for patients with atrial fibrillation (AF) who have undergone a successful transcatheter aortic valve implantation (TAVI) and have been discharged home.
The risk score is a product of the ENVISAGE-TAVI AF...
Medtronic has announced that it has completed the acquisition of Affera. The acquisition will expand Medtronic’s cardiac ablation portfolio to include its first cardiac mapping and navigation platform.
Among Affera’s offerings is the Affera Prism-1 cardiac mapping and navigation platform,...
Galaxy Medical has announced that it received the CE mark for its Centauri pulsed electric field (PEF) system and launched its commercial programme.
The approval allows the company to market the system for the treatment of paroxysmal atrial fibrillation (AF)...
Medtronic has announced that its investigational EV ICD system—a defibrillator with the lead placed under the breastbone, outside of the heart and veins—achieved a defibrillation success rate of 98.7% and met its safety endpoints in a global clinical trial.
Findings...
Screening for atrial fibrillation (AF) using a smartphone app more than doubled the detection and treatment rate in older people compared to routine screening, researchers behind the eBRAVE-AF trial have reported at the European Society of Cardiology (ESC) annual...
Results of one of the first and only randomised, blinded trials to assess the use of an artificial intelligence (AI) algorithm as an assessment tool for cardiac function suggest that AI improves evaluation when compared to assessment by a...
New guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death have been published today in the European Heart Journal and call for more automated external defibrillators (AEDs) to be placed in public...
Merit Medical has announced the launch of its SafeGuard Focus Cool compression device.
It is part of a broader cardiac portfolio that offers products and accessories necessary for cardiac rhythm management, electrophysiology, and lead extraction procedures as well as interventional...
Thermedical has announced that the US Food & Drug Administration (FDA) has approved an open-label, single-arm interventional clinical trial to evaluate the safety and efficacy of the Thermedical SERF ablation system with the Durablate catheter in people with ventricular...
Cannabis prescribed for chronic pain is associated with an elevated risk of heart rhythm disorders, according to research presented at the European Society of Cardiology (ESC) Congress (ESC 2022, 26–29 August, Barcelona, Spain).
Study author Nina Nouhravesh of Gentofte University...
Patients with recently diagnosed atrial fibrillation (AF) with a high burden of comorbidities should be considered for early rhythm control to reduce cardiovascular outcomes, whereas those with fewer comorbidities may have less favourable outcomes with this strategy.
These are among...
The National Institute for Health and Care Excellence (NICE) has issued draft guidance recommending KardiaMobile 6L for measuring cardiac QT interval in people having antipsychotic medication.
People taking antipsychotic medication may need testing for heart problems before starting treatment and...
A new scientific statement from the American Heart Association (AHA) provides guidance about sleep-disordered breathing and its association with the development of cardiac arrhythmia.
Published in Circulation, the analysis was authored by a panel led by Reena Mehra and Mina...
CathVision has secured US$7.2 million in funding from existing investors to help advance commercial operations driving adoption of the ECGenius system, the company's EP recording technology.
Funding will also support the continued development of artificial intelligence-powered analytic modules to provide...
A first-in-human multicentre trial using in-catheter, heated saline-enhanced radio frequency (SERF) ablation for patients with ventricular tachycardia (VT) has reported that the technique showed complete acute and satisfactory mid-term control of difficult VTs, which had failed one to five...
OSYPKA AG, a medical device company based in Rheinfelden, Germany, has received the CE mark under the European Union’s Medical Device Regulation (MDR) for a class III cardiac pacing electrode.
“This is a new milestone that is also valuable for...
The Heart Rhythm Society (HRS) has announced the full line-up of speakers and sessions for its first annual HRX meeting (8–10 September, San Diego, USA).
The conference offers clinicians, engineers, product developers, investors, entrepreneurs, non-profit organisations, and patient advocacy groups...
Projected rates of cardiovascular risk factors and disease will increase significantly in the USA by 2060, a study published in the Journal of the American College of Cardiology (JACC) has claimed.
Substantial increases in cardiovascular trends may contribute to a...
A project to develop genetic therapies to effectively cure cardiomyopathy has been announced as the winner of the British Heart Foundation’s Big Beat Challenge, a £30 million innovation project targeting unmet needs in heart disease.
CureHeart—a project involving researchers from...
Adagio Medical has announced the publication of the results of its Cryocure-2 ultra-low temperature cryoablation (ULTC) study in patients with atrial fibrillation (AF) in the Journal of American College of Cardiology (JACC), Clinical Electrophysiology.
The study prospectively enrolled 79 patients...
InCarda Therapeutics has announced enrolment of the first patient in the company’s pivotal phase 3 RESTORE-1 clinical trial of InRhythm (orally inhaled flecainide) in patients with newly-diagnosed atrial fibrillation (AF) or recurrent paroxysmal atrial fibrillation (PAF).
At an end-of-phase 2...
Acutus Medical has announced approval of its AcQMap high resolution imaging and mapping system and the AcQMap 3D imaging and mapping catheter in Japan.
Acutus’ International Alliance partner Biotronik has commenced training and early market development activities in collaboration with...
iRhythm Technologies has received US Food and Drug Administration (FDA) 510(k) clearance for its ZEUS (Zio ECG Utilization Software) system for the Zio Watch.
Produced in partnership with Verily, the ZEUS system combines deep learned algorithms with a cardiac arrhythmia...
Acutus Medical has announced that David Roman has been appointed president and chief executive officer, and member of the Board of Directors, effective immediately. The company also announced preliminary second quarter revenue results.
Roman, who joined the company as chief...
Around 80% of patients diagnosed with atrial fibrillation (AF) in the UK population are eligible for early rhythm control, an analysis of the EAST-AFNET 4 trial, published in Heart, has found.
Rhythm control therapy is typically delayed unless patients have...
Boston Scientific is collaborating with Telefónica España to provide ‘advanced healthcare technology’, including 5G, through its recently launched Remote Clinical Support Centre—RhythmCARE—which is intended to provide remote support to a number of hospitals in Europe, Africa and the Middle...
A research project at the University of Leeds (Leeds, UK) aiming to find an easier way to establish the optimum heart rate for heart failure patients with pacemakers, has received a £200,000 grant from Heart Research UK.
According to researchers,...
Stereotaxis has announced the CE mark submission for its MAGiC catheter, a robotically navigated magnetic interventional ablation catheter for minimally invasive cardiac ablation procedures.
MAGiC is used in conjunction with Stereotaxis’ robotic systems and designed to provide catheter precision, stability...
Abbott has introduced its EnSite X EP a cardiac mapping platform with EnSite Omnipolar Technology (OT) in Canada.
The platform allows for the creation of a 3D model of the patient’s cardiac anatomy in real-time, improving how physicians identify and...
Biotronik’s LiveSupport feature was used to remotely support two Biomonitor implantable cardiac monitor (ICM) implants shortly after launch. Both procedures were performed with a Biotronik representative in a remote location.
Developed in part as a response to the COVID-19 pandemic,...
Catheter ablation of atrial fibrillation (AF) was economically attractive compared with drug therapy in the CABANA (Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation) trial, investigators have reported in Circulation.
CABANA was an investigator-initiated, open-label, multicentre, randomised trial in...
Cardiac testing company CardiNor AS has announced that it has signed an exclusive agreement with IBL-America for sales of the CardiNor Secretoneurin ELISA test in the USA.
By initially targeting research institutes, clinical research organisations and pharmaceutical companies, the company...
HeartBeam is expanding the available patient population for its emergency department software technology solution. In evaluating the electrocardiogram (ECG) database for the clinical validation of HeartBeam’s platform technology, a significant portion of consecutive patients fell into the category of...
Acutus Medical has announced the completion of the first of two closings for its previously disclosed sale of the company’s left-heart access portfolio to Medtronic.
Additionally, Acutus Medical has also announced entry into a new longer-term credit facility with Deerfield...
People who develop an arrhythmia after surgery have an increased risk of subsequently being admitted to hospital with heart failure, according to a study of over three million patients published in the European Heart Journal. The study showed that...
Acutus Medical has announced the commercial launch of an expanded suite of left-heart access products to now include the AcQCross Qx system for use with the TruSeal and FXD delivery system for the Watchman left atrial appendage closure (LAAC)...
In patients treated with pulsed field ablation (PFA) for symptomatic paroxysmal atrial fibrillation (AF), the incidence of asymptomatic thromboembolic cerebral events or lesions detected by magnetic resonance imaging (MRI) was as low as 3%. This is according to the...
A study, published in the European Heart Journal–Digital Health, shows the predictive potential of a deep-learning model in identifying patients at risk of atrial fibrillation (AF) following monitoring with a 24-hour ambulatory electrocardiogram (ECG), despite no documented prior AF,...
Recommendations on how to use gene testing to prevent sudden cardiac death in athletes and enable safe exercise have been published in the European Journal of Preventive Cardiology.
“Genetic testing for potentially lethal variants is more accessible than ever before...
A national, multicentre registry investigating the safety and efficacy of laser lead extraction has reported high success- and low procedure-related complication rates associated with the procedure. This is the headline finding of the GALLERY (German laser lead extraction gallery)...
Conformal Medical has announced the launch of the CONFORM pivotal trial with the enrolment of the first patients at two US sites.
The investigational device exemption (IDE) study will evaluate the safety and efficacy of the company's CLAAS system compared...
Dual antiplatelet therapy (DAPT) after left atrial appendage occlusion (LAAO) using the Watchman FLX (Boston Scientific) device was comparable in terms of rates of death, stroke, bleeding or device-related thrombus when compared to use of aspirin with an anticoagulant,...
Incidence of ventricular arrhythmias can be linked to days when air pollution is high, according to research presented at Heart Failure 2022 (21–24 May, Madrid, Spain). The study was conducted in patients with an implantable cardioverter defibrillator (ICD), enabling...
AtriCure has announced that the first patient has been treated in the HEAL-IST trial, evaluating the safety and effectiveness of AtriCure’s Isolator Synergy Clamp for the treatment of drug-refractory patients diagnosed with inappropriate sinus tachycardia (IST). The first patient...
Abbott has announced that the US Food and Drug Administration (FDA) has approved the Aveir single-chamber (VR) leadless pacemaker for the treatment of patients in the US with slow heart rhythms.
The Aveir leadless pacemaker is implanted directly inside the...
A study has revealed the “global collateral damage” caused by the disruption to cardiac services caused by the COVID-19 pandemic. Writing in the European Heart Journal, researchers warn that problems with heart health will “...continue to accrue unless mitigation...
The use of a subcutaneous-implantable defibrillator (S-ICD, Boston Scientific) reduced the rate of major, lead-related complications by as much as 92% when compared to a transvenous device (TV-ICD), according to the investigators of the ATLAS trial, who presented their...
Findings of the US multicentre cardioneural ablation (CNA) registry were presented during a late-breaking clinical trial session at the Heart Rhythm Society’s 2022 annual meeting (HRS 2022, 29 April–1 May, San Francisco, USA), showing that ablation can be a...
Attune Medical has announced that the first patients have been enrolled in the IMPACT II— Improving Oesophageal Protection During Catheter Ablation for Atrial Fibrillation—study, evaluating the use of the ensoETM in cardiac radiofrequency ablation procedures.
Data from this study will...
inHEART has received US Food and Drug Administration (FDA) 510(k) clearance for its inHEART Models software suite that enables 3D visualisation and analysis of anatomical structures for pre-procedural planning and intraprocedural use.
With this clearance, inHEART will expand its commercial...
Endotronix has announced data from the SIRONA 2 clinical trial evaluating safety and efficacy of its Cordella pulmonary artery pressure sensor system in New York Heart Association (NYHA) class III heart failure patients.
The prospective, multicentre trial met all primary...
Findings of the EAST-AFNET 4 trial, examining the benefit of early rhythm control therapy in patients with newly diagnosed atrial fibrillation (AF), should influence future guidelines for the treatment of AF patients.
This was the message of Paulus Kirchhof (Hamburg,...
Results of a study presented at the Heart Rhythm Society’s 2022 annual meeting (HRS 2022, 29 April–1 May, San Francisco, USA) show that the success of intended same day discharge (SDD) improves over time for patients undergoing atrial fibrillation...
Use of an artificial intelligence (AI)-based app using electrocardiogram (ECG) signals recorded with an Apple Watch is able to identify left-ventricular dysfunction, research presented at the Heart Rhythm Society’s 2022 annual meeting (HRS 2022, 29 April–1 May, San Francisco,...
CathVision has announced US Food and Drug Administration (FDA) 510(k) clearance of the ECGenius electrophysiology recording system. ECGenius acquires high-fidelity, low-noise, cardiac electrograms to help electrophysiologists improve the diagnosis and treatment of complex atrial arrhythmias, including atrial fibrillation (AF).
Conventional...
Ceryx Medical has raised £3.8m in seed funding which will be used to commercialise its technology and commence the first-in-human clinical study of the cardiac rhythm management device, Cysoni, later this year.
The new funding involves Icehouse Ventures, The Development...
The Heart Rhythm Society (HRS) has announced the findings of three clinical trials demonstrating positive outcomes of conduction system pacing (CSP) for patients in need of cardiac resynchronisation therapy (CRT). The studies were presented as late-breaking clinical science at...
Results from a new clinical trial found aggressive blood pressure (BP) control reduced the risk of left-ventricular conduction disease. This study is the first to provide causal evidence that cardiac conduction disease is preventable, according to researchers. Findings were...
Abbott has announced US availability of its Amplatzer steerable delivery sheath, which is used with the company's Amplatzer Amulet left atrial appendage (LAA) occluder to treat people with atrial fibrillation (AFib) who are at risk of ischaemic stroke.
For patients...
Medtronic has announced new data from the STROKE AF clinical trial, showing that large and small vessel ischaemic stroke patients who receive short-term or intermittent monitoring using holter monitors and 30-day external monitors may not be optimally managed for...
CathVision has completed patient enrolment in the CathVision ECGenius System clinical evaluation study at the University of Vermont Medical Center (Burlington, USA). The study is the first in the USA to evaluate the safety and technical performance of ECGenius...
Medtronic has announced that Laura Mauri has been appointed as the company’s chief scientific, medical and regulatory officer. This appointment adds to Mauri's prior responsibilities as chief clinical and regulatory officer, aligning and integrating the company's scientific, medical, clinical research...
The use of high-power, short duration ablation for atrial fibrillation (AF) has the potential to speed up procedural times and increase the number of ablations that can be performed in a day.
This is the message of Teresa Strisciuglio (Clinica...
The pandemic saw a boom in the use of remote technologies and telehealth for patient monitoring and management, including for patients with heart failure—and many of these technologies look to have a role in the future, even despite a...
Medtronic has announced two AccuRhythm AI algorithms will be applied to LINQ II insertable cardiac monitors (ICM) through cloud-based updates in Europe later this spring.
AccuRhythm AI applies artificial intelligence (AI) to heart rhythm event data collected by LINQ II,...
Fitbit has received clearance from the US Food and Drug Administration (FDA) for a new photoplethysmography (PPG) algorithm to identify atrial fibrillation (AF). The algorithm will power a new irregular heart rhythm notification feature on Fitbit.
In a press release,...
Cardiologs has announced the results of a clinical study showing that its deep learning artificial intelligence (AI) software reduces inconclusive results returned by the latest Apple Watch ECG companion app—Apple ECG 2.0—while maintaining performance (sensitivity and specificity).
“Wearable devices, such...
Among the array of remote monitoring and digital arrhythmia management tools on display at the 2022 European Heart Rhythm Association annual meeting (EHRA 2022, 3‒5 April, Copenhagen, Denmark), Boston Scientific showcased its Heart Connect remote support system, which has...
FEops has announced that it received authorisation from the US Food and Drug Administration (FDA) for FEops Heartguide pre-operative planning of left atrial appendage occlusion (LAAO) with Abbott’s Amplatzer Amulet and Boston Scientific’s Watchman FLX device.
FEops Heartguide is a...
iRhythm Technologies has announced the results of three clinical research studies presented at the American College of Cardiology’s annual scientific sessions (ACC 2022, 2–4 April, Washington DC, USA).
According to iRhythm, the evidence further validates the Zio service as a...
Abbott has announced that the US Food and Drug Administration (FDA) has approved the Aveir single-chamber (VR) leadless pacemaker for the treatment of patients in the USA with slow heart rhythms. This marks significant advancement for patient care and...
Screening for atrial fibrillation (AF) should be integrated into flu vaccination and cancer screening programmes, according to a survey of general practitioners (GPs) conducted by the AFFECT-EU project and presented at the 2022 European Heart Rhythm Association annual meeting...
A simple electrocardiogram (ECG) can pinpoint hospitalised COVID-19 patients at high risk of death who might need intensive management, according to the authors of a study presented at the 2022 European Heart Rhythm Association annual meeting (EHRA 2022, 3‒5...
Overweight patients with atrial fibrillation (AF) are more likely to experience a return of the heart rhythm disorder after a corrective procedure than those of normal weight, according to research presented at the 2022 European Heart Rhythm Association annual...
Results of a large-scale, real-world analysis of US Centers for Medicare & Medicaid Services (CMS) data on the rates of guideline adherence and associated mortality in patients with cardiac implantable electronic device (CIED) infection show that only around one...
Use of cardiac magnetic resonance (CMR) may relevant role of CMR in risk stratification for sudden cardiac death after myocardial infarction, to late-breaking research presented at the 2022 European Heart Rhythm Association annual meeting (EHRA 2022, 3‒5 April, Copenhagen,...
Recommendations on the prevention and management of interference caused by medical procedures in patients with implanted electronic cardiac devices has been published today in EP Europace, and presented at the 2022 European Heart Rhythm Association annual meeting (EHRA 2022,...
The “exponential growth” in the use of digital devices for monitoring arrhythmias has led to the development of a new international consensus document. The paper, published today in EP Europace, will also be presented at the 2022 European Heart...
East End Medical has announced the first commercial use of its SafeCross transseptal radiofrequency (RF) puncture and steerable balloon introducer system. The system is designed to provide a predictable and safe solution for performing electrophysiology and structural heart interventions...
The launch of the latest KODEX-EPD cardiac mapping and navigation system is among the features Royal Philips will showcase at the 2022 European Heart Rhythm Association annual meeting (EHRA 2022, 3‒5 April, Copenhagen, Denmark), the company has revealed in...
GE Healthcare and AliveCor have announced a partnership to deliver medical-grade six-lead electrocardiograms (ECGs) taken by patients on an AliveCor KardiaMobile 6L ECG device outside of the hospital setting directly into GE Healthcare’s MUSE Cardiac Management System for physicians...
Researchers have developed a clinical algorithm that distinguishes between treatable sudden cardiac arrest and untreatable forms of the condition.
The findings, Journal of the American College of Cardiology: Clinical Electrophysiology, have the potential to enhance prevention of sudden cardiac arrest,...
Black and Hispanic individuals experiencing a cardiac arrest either at home or in public are substantially less likely to receive cardiopulmonary resuscitation (CPR) from a bystander compared to white individuals, according to a study presented at the American College...
Researchers want to leverage the centuries-old art of cutting paper into designs to develop a sensor sheet that can stretch and breathe alongside the skin while collecting electrocardiographic data, according to a press review. In Applied Physics Reviews, the...
Stereotaxis has announced that Poland’s National Institute of Cardiology in Warsaw has established the first robotic electrophysiology programme in the country with Stereotaxis’ Genesis robotic magnetic navigation (RMN) system. The National Institute of Cardiology is the only hospital in...
Research published in the journal BMC Medicine investigates the impact of kidney function in patients on antithrombotic therapy.
The study, a post hoc sub-group analysis focusing on recurrent bleeding events in the AFIRE (Atrial Fibrillation and Ischemic Events with Rivaroxaban...
Pulmonary vein-left atrial (PV/LA) volume ratio is a significantly better predictor power for atrial fibrillation (AF) recurrence than LA dimension in patients with normal or mild LA enlargement, with an association between PV/LA volume and the PITX2 gene. This...
A consensus document outlining design principles and outcome definitions for device-based therapies for hypertension has been published in the journal Circulation.
The development of the document has been led by the Hypertension Academic Research Consortium (HARC), which aims to create...
Pulsed-field ablation (PFA) and/or radiofrequency ablation (RFA) using a lattice-tip catheter can both isolate pulmonary veins (PVs) and create linear lesions efficiently and safely, with a high degree of lesion durability, and promising freedom from arrythmia recurrence. This was...
DiA Imaging Analysis Ltd, announced today a multi-year agreement with Change Healthcare. Change Healthcare's proven cardiology solutions, along with DiA's AI-based algorithms, will benefit echocardiologists and other imaging specialists by improving the efficiency of routine tasks and the effectiveness...
A new strategy using telehealth to monitor blood pressure at home for several months immediately after a stroke had a positive impact on patient engagement and blood pressure control among people who live in historically under-resourced communities, according to preliminary research presented at...
Hospitals in England are trialling remote monitoring technology from iRhythm Technologies—Zio XT—which is designed to aid the detection of arrhythmias supported by artificial intelligence.
Typically patients experiencing symptoms of an arrhythmia would need to go to hospital for 24 hours...
Royal Philips has announced new research evaluating mobile cardiac outpatient telemetry (MCOT) as a first-line diagnostic ambulatory monitoring solution with post-cryptogenic stroke patients.
The study determined that a 30-day continuous monitoring program using the Philips BioTel Heart MCOT patch, followed...
As a result of the COVID-19 pandemic, the increasing uptake of paediatric electrophysiology (EP) telehealth services has demonstrated clear ability to deliver healthcare for a pathologically and geographically diverse group of patients, who mostly report satisfaction with the service....
Portable electronic devices containing magnets may inhibit pulse generators for implanted cardiac devices (ICDs) and pacemakers according to new research published in Circulation: Arrhythmia and Electrophysiology.
The authors of the research, Corentin Féry (Institute for Medical Engineering and Medical Informatics,...
A recent systematic review and meta-analysis, published in the Journal of Interventional Cardiac Electrophysiology, has deemed same-day discharge following catheter ablation of atrial fibrillation (AF) to be safe and feasible.
According to a recent review, it is common practice...
Results from a new analysis assessing real-world outcomes with the Watchman FLX (Boston Scientific) left atrial appendage closure (LAAC) demonstrated a low rate of major adverse events at seven days following implant of the device.
The SURPASS analysis, which included...
Women remain underrepresented in leading cardiovascular clinical trials, corresponding with underrepresentation and a lack of gender diversity among presenters at scientific meetings, according to a review published in the Journal of the American College of Cardiology (JACC).
A previous analysis...
Wearable technology and digital health specialist Activinsights has announced a partnership agreement with the cardiovascular research organisation Cardialysis to promote the use of remote monitoring wearables as a tool for researchers to better understand the daily lifestyles of cardiac...
A review paper published in JACC: Clinical Electrophysiology explores the potential mechanisms linking stress and atrial fibrillation (AF), and the possible uses of stress reduction in the management of the condition.
Stress has been linked with poor health outcomes, though...
Historically, catheter ablation has been reserved for atrial fibrillation (AF) patients who have failed to respond to, or tolerate antiarrhythmic drug therapy, electrophysiologist Dhiraj Gupta (Liverpool Heart and Chest Hospital, Liverpool, UK) tells Cardiac Rhythm News. However, he explains,...
Both cryoballoon ablation (CBA) and hot balloon ablation (HBA) are effective treatment options for atrial fibrillation (AF), however, patients treated with HBA are more likely to receive touch-up ablation, with a longer procedure time. This was the main concluding...
Siemens Healthineers has announced the launch of Artis icono biplane—an angiography system with detectors optimised in size for integrated use in the cath lab.
The system offers new features for diagnosing and treating cardiac arrhythmia, coronary heart disease, and structural...
Abbott has announced that the US Food and Drug Administration (FDA) has approved an expanded indication for the company’s CardioMEMS HF system to support the care of more people living with heart failure.
With the expanded indication, an additional 1.2...
Medtronic has announced that the Freezor and Freezor Xtra cardiac cryoablation catheters are approved by the US Food and Drug Administration (FDA) to treat the growing prevalence of paediatric atrioventricular nodal reentrant tachycardia (AVNRT).
AVNRT is the most common form...
Fewer than half of UK citizens are aware of the link between arrhythmia and stroke, while around one in five people with a personal or familial risk of heart disease were unaware of how to check their pulse to...
Cardiologist and researcher Robert Califf has been confirmed as the commissioner of the US Food and Drug Administration (FDA). Califf returns to the role that he held between September 2016 and January 2017, having been confirmed in a vote...
A new timely practice—known as the “1-2-3-4-day” rule—for the administration of direct oral anticoagulants (DOACs) in patients after acute ischaemic stroke or transient ischaemic attack (TIA) is deemed feasible to decrease the risk of recurrent stroke, systemic embolism, and...
Boston Scientific has announced the close of its acquisition of Baylis Medical Company, a company that offers advanced transseptal access solutions as well as guidewires, sheaths and dilators used to support catheter-based left-heart procedures.
“The close of this acquisition allows...
Vektor Medical has announced that the University of California, San Diego Health (UCSD, San Diego, USA) is the first hospital system to offer the vMap arrhythmia mapping system.
The recently US Food and Drug Administration (FDA)-approved technology identifies potential arrhythmia...
CoreMap has announced the closure of a US$3 million series A milestone financing, with a US$20 million series B financing, which includes a diverse group of strategic, institutional, venture, and individual partners.
Pierre Jaïs of the Electrophysiology and Heart Modeling...
A collaboration between Ceryx Medical Limited and the scientists at the Auckland Bioengineering Institute (ABI) and the Universities of Bath and Bristol, have developed technology that they claim, could improve the outlook for patients with serious heart conditions.
Ceryx Medical's...
atHeart Medical has announced that it has received US Food and Drug Administration (FDA) approval for the start of the second phase of its ASCENT ASD US investigational device exemption (IDE) pivotal trial, evaluating the safety and efficacy of...
The placement of an interarterial shunt device—the Corvia atrial shunt (Corvia Medical)—did not reduce the total rate of heart failure events in a population of patients with heart failure with preserved or mildly reduced ejection fraction (HFpEF or HFmrEF).
This...
Abbott has announced the first patient implants of a dual-chamber leadless pacemaker system as part of its AVEIR DR i2i pivotal clinical study. The implant of Abbott's investigational Aveir dual-chamber leadless pacemaker represents a technological milestone for leadless pacing...
BioCardia has announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the CardiAMP cell therapy system for the treatment of heart failure.
CardiAMP cell therapy uses autologous bone marrow cells delivered to the heart...
A same-day discharge strategy for left atrial appendage occlusion (LAAO) could become the standard approach for this technique, according to the authors of a study published in the Journal of Invasive Cardiology, who concluded that the strategy is safe,...
AliveCor has announced the launch of KardiaMobile Card—an electrocardiogram (ECG) device the size of a standard credit card.
“After disrupting traditional ECG monitoring with our game-changing Kardia platform, we have now achieved the unprecedented milestone of creating the first-ever credit-card-sized...
Use of temperature-controlled ablation for the treatment of atrial fibrillation (AF) is effective and highly efficient, according to Jose Osorio—the director of electrophysiology at Grandview Medical Center in Birmingham, Alabama, USA. His comments follow the completion of a US-based...
The US Food and Drug Administration (FDA) has issued two final guidances providing recommendations for including patient perspectives in medical device clinical studies.
As per an FDA press release, the finalised version of the first of these two guidance...
Royal Philips has announced the introduction of a full-service, at-home, 12-lead electrocardiogram (ECG) solution for use in decentralised clinical trials. The clinical-grade solution is the most advanced patient-centric ECG offering within the company’s cardiac monitoring portfolio, pairing data readings...
A recent study has demonstrated 100% pulmonary vein isolation (PVI) using only pulsed field ablation (PFA), resulting in no PFA-related serious adverse events. This was the concluding finding of the PULSED AF (Pulsed-field ablation to irreversibly electroporate tissue and...
Contact-force (CF)-guided ablation to treat typical atrial flutter does not reduce recurrent atrial arrhythmia at 12 months follow-up, in comparison to ablation blinded for contact force. This was the main conclusion of a randomised controlled trial (RCT), findings of...
Acute alcohol consumption is associated with a higher risk of atrial fibrillation (AF), a study published in Nature Cardiovascular Research has concluded
The study is the first to show an association between increased drinking and hospital visits for AF in...
Royal Philips is supporting the American Heart Association’s (AHA’S) effort to generate awareness among cross-disciplinary specialties and improve survival rates from cardiovascular implantable electronic device (CIED) infections.
With the support of Philips, the American Heart Association’s national CIED infection initiative...
According to a press release, AtriAN Medicals clinical data from the Neural atrial fibrillation (AF) study was presented by Vivek Reddy (Mount Sinai Hospital, New York, USA) at this year's renowned annual AF symposium 2022 (13—15 January, New York...
MicroPort CRM has recently received the pharmaceuticals and medical devices agency (PMDA) Japanese regulation agency approval for the Alizea range of portable pacemakers. The devices are equipped with bluetooth technology for streamlined remote monitoring when paired with MicroPort CRM's...
Abbott has received clearance from the US Food and Drug Administration (FDA) for the EnSite X EP system with EnSite Omnipolar Technology (OT), a new cardiac mapping platform available in the USA and across Europe that is designed to...
Medtronic has received approval from Japan's Ministry of Health, Labor and Welfare for the sale and reimbursement of the Micra AV Transcatheter Pacing System (TPS), and the company will launch the product this month.
This approval expands the number of...
Medtronic has announced that it has entered into an agreement to acquire medical technology company Affera.
Affera designs and manufactures cardiac mapping and navigation systems and catheter-based cardiac ablation technologies, including a differentiated, focal pulsed field ablation (PFA) solution, for...
Khaldoun Tarakji, associate section head of cardiac electrophysiology at the Heart, Vascular, and Thoracic Institute at Cleveland Clinic (Cleveland, USA) has joined Medtronic as vice president and chief medical officer (CMO) of the company's Cardiac Ablation Solutions (CAS) Operating...
The National Institute for Health and Care Excellence (NICE) has issued Medical Technologies Guidance (MTG) recommending KardiaMobile (AliveCor) as an option for detecting atrial fibrillation (AF) in patients with suspected paroxysmal AF, who present with symptoms such as palpitations...
Endotronix has announced that the US Food and Drug Administration (FDA) has granted approval for an amendment to the company's PROACTIVE-HF study, a pivotal Investigational Device Exemption (IDE) trial investigating the Cordella Pulmonary Artery (PA) Pressure Sensor, shifting the...
Adagio Medical has announced first-in-human cases of pulsed field cryoablation (PFCA) for the treatment of atrial fibrillation (AF).
The cases were performed at Medicover Hospital, Warsaw, Poland by Pawel Derejko, head of the Department of Cardiology at Medicover and Atul...
Volta Medical has announced the introduction of VX1 artificial intelligence (AI) software at three US hospitals. New York Presbyterian Queens, Northwell Health’s Lenox Hill Hospital both in New York, and Ascension St. Vincent’s Riverside, Jacksonville, have been named as...
Doctors at St David's Medical Center in Austin, USA recently implanted a new neurostimulator technology in patients to help treat advanced heart failure—and are among the first physicians in the USA to do so, according to a press release.
The...
Affera has announced that the first patient was treated in the recently approved SPHERE PerAF trial, a US Food and Drug Administration (FDA) Investigational Device Exemption (IDE) pivotal randomised trial, to evaluate the safety and effectiveness of the Affera...
FAST BioMedical has announced the publication of additional results from the EMPAKT CHF (Estimating versus Measuring Plasma Volume and Kidney Function in Acute Decompensated Congestive Heart Failure) clinical trial in the European Society of Cardiology (ESC) Heart Failure journal....
Kardium has announced the completion of successful first-in-human (FIH) procedures with the Globe pulsed field system.
Vivek Reddy of Mount Sinai Hospital (New York, USA) and Petr Neužil of Na Homolce Hospital (Prague, Czech Republic), performed the procedures in Prague,...
Implicity has announced US Food and Drug Administration (FDA) clearance for a novel medical algorithm that analyses ECG data from implantable loop recorders (ILRs).
“This is an important step for Implicity as we expand in the US market,” said Arnaud...
LifeTech Scientific Corporation has announced that it has extended its agreements with Medtronic to further the strategic collaboration on the HeartTone domestic pacemaker project and to start the collaboration on domestically-made MRI-conditional pacemakers. Together with MRI-conditional leads, these products...
Stereotaxis has announced the publication of the results of a prospective, multicentre study comparing the incidence of silent cerebral embolism (SCE) in atrial fibrillation (AF) patients undergoing robotic cardiac ablation or manual in Frontiers in Cardiovascular Medicine.
The study evaluated...
New research suggests that implantable cardioverter defibrillators (ICDs) are not used as frequently for females and patients of colour, despite the increased use of ICDs in the management of patients with hypertrophic cardiomyopathy (HCM), over recent decades.
In patients with HCM,...
Royal Philips has announced an expansion of its ultrasound portfolio with new imaging tools and features for cardiology, designed to “increase diagnostic confidence and workflow efficiency”. Philips will be showcasing these developments through its participation in the EuroEcho 2021...
SyMap Medical has announced the completion of a financing round which will be used to accelerate global clinical trials for the company’s renal mapping and selective renal denervation systems.
The financing round of nearly US$100 million was led by Primavera...
One in 10 junior doctors training to be cardiologists in the UK say they have been bullied, the results of a survey published online in the journal Heart indicate. Women and those who qualified in medicine outside the UK...
Restore Medical has announced preliminary results of the first-in-human clinical trial of ContraBand, a breakthrough treatment for patients suffering from congestive heart failure. The minimally-invasive, catheter-delivered implants are used to treat patients with chronic left ventricular failure.
ContraBand has been...
Boston Scientific has announced the start of the MODULAR ATP clinical trial to evaluate the safety, performance and effectiveness of the mCRM modular therapy system. The mCRM system consists of two cardiac rhythm management (CRM) devices intended to work...
Persistent opioid use following a cardiac implantable electronic device (CIED) procedure is common, according to a study published in Circulation, which reports that 12% of patients receiving a CIED are consistently using pain medication in the months after the...
Canon Medical has announced the introduction of the Precise IQ Engine (PIQE) deep-learning reconstruction (DLR) and SilverBeam filter.
PIQE, which Canon describes as super-resolution deep-learning reconstruction technology for cardiac computed tomography (CT) scans, delivers “exceptional” cardiac CT image quality by...
Acutus Medical has announced the initiation of AcQForce PFA-CE, a clinical study that will evaluate the safety and performance of the company’s focal force sensing pulsed field ablation (PFA) catheter and system in combination with its novel noncontact 3D...
Cardiologs announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) to use its AI-powered cardiac diagnostics platform for paediatric cardiology.
The expanded authorisation was granted based on an analysis of the company's improved deep...
The advent of pulsed field ablation (PFA) will revolutionise the treatment of atrial fibrillation (AF), Tom de Potter (Aalst, Belgium) tells Cardiac Rhythm News, discussing the future of the treatment of the condition.
PFA utilises a controlled electric field to...
European Society of Cardiology (ESC) guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic has been published in the European Heart Journal.
The guidance brought together a large number of key opinion leaders at the outset...
Cardialen has received approval from the US Food and Drug Administration (FDA) for an investigational device exemption (IDE) to begin a clinical trial of its Multipulse Therapy (MPT) to treat paroxysmal and persistent atrial fibrillation (AF).
MPT is delivered as...
Women diagnosed with breast cancer are significantly more likely to develop atrial fibrillation (AF) compared to women without cancer, according to research published in the European Heart Journal. These patients have a three-fold increased risk of death from either heart...
Results of the Fitbit Heart study, presented during a late-breaking science session at the American Heart Association’s Scientific Sessions 2021 (AHA 2021; 13–15 November; virtual), suggest that the consumer fitness tracking watch, Fitbit, was capable of detecting undiagnosed atrial...
HeartVista has received US Food and Drug Administration (FDA) 510(k) clearance to deliver its AI-assisted One Click magnetic resonance imaging (MRI) acquisition software on Siemens Healthineers MRI scanners.
The use of cardiac MRI, also known as cardiac magnetic resonance (CMR),...
Ahead of the American Heart Association’s 2021 annual scientific sessions (AHA 2021, 13–15 November, virtual), Cardiac Rhythm News speaks to the meeting’s programme committee chair Manesh Patel (Duke University School of Medicine, Durham, USA) about some of the highlights...
Data from the global Leadless II IDE study evaluating the Aveir (Abbott) leadless pacemaker in patients with certain abnormal heart rhythms show that the device met its prespecified primary safety and efficacy endpoints .
The findings were presented today in...
Adults with type 1 or type 2 diabetes and atrial fibrillation (AF) are less likely to notice irregular heartbeat symptoms, more likely to have a lower quality of life (QoL), and experience more co-existing health conditions than people with...
Caption Health and Ultromics have announced a strategic partnership to accelerate cardiovascular disease detection and treatment for more patients in more accessible care settings. Together, the companies will jointly offer the Caption AI software platform alongside Ultromics’ EchoGo deep...
Vektor Medical has announced US Food and Drug Administration (FDA) 510(k) clearance for its novel computational electrocardiogram (ECG) mapping system, vMap.
vMap is designed to map potential arrhythmia sources associated with stable or unstable arrhythmias anywhere in the heart—including all...
Patients who went into cardiac arrest in the cardiac cath lab were more likely to survive to hospital discharge than those who had a cardiac arrest in the intensive care unit (ICU), yet less likely to survive than those...
Medtronic has announced its ambition to achieve net zero carbon emissions by fiscal year 2045 across its operations and value chain to accelerate efforts to combat climate change. The announcement comes amidst the 2021 United Nations Climate Change Conference...
Royal Philips has signed an agreement to acquire Cardiologs, a developer of cardiac diagnostics using artificial intelligence (AI) and cloud technology. Cardiologs will further strengthen Philips’ cardiac monitoring and diagnostics offering with innovative software technology, electrocardiogram (ECG) analysis and...
Findings of the SWISS-APERO trial, presented at the Transcatheter Cardiovascular Therapeutics annual meeting (TCT 2021, 4–6 November, Orlando USA and virtual), comparing the Amulet (Abbott) and Watchman FLX (Boston Scientific) left atrial appendage closure (LAAC) devices, show similar rates...
Left atrial appendage occlusion (LAAC) remains non-inferior to non vitamin-K-antagonists (NOACs) for the prevention of major cardiovascular, neurological or bleeding events in patients with non-valvular atrial fibrillation (AF) at long-term follow-up.
These are four-year findings of the PRAGUE-17 clinical trial,...
South Korean artificial intelligence (AI) developer Vuno has announced that the Korean Ministry of Food and Drug Safety (KFDA) has designated the company's AI-based electrocardiogram (ECG) analysis software Vuno Med-DeepECG as a breakthrough device. Using deep learning, the software...
Researchers from Massachusetts General Hospital (MGH, Boston, USA) have reported the detection and characterisation of thrombosis using fibrin-targeted positron emission tomography and magnetic resonance in a first-in-human study published in JACC: Cardiovascular Imaging.
Atrial fibrillation (AF) can cause clots to...
Research presented at the American College of Rheumatology’s annual meeting (ACR Convergence, 3–9 November, virtual) shows that hydroxychloroquine does not appear to be associated with QTc interval prolongation, an irregularity of the electrical activity of the heart that places...
A pragmatic clinical trial—CHANGE AFib—will determine whether early treatment with the antiarrhythmic drug dronedarone improves cardiovascular and long-term outcomes in patients presenting with first-detected atrial fibrillation (AF). The trial represents a collaboration between the American Heart Association (AHA) and...
CathVision has announced the first patient enrolments in the PVISION multicentre clinical study, investigating the automated assessment of pulmonary vein isolation (PVI) using the company's artificial intelligence (AI) algorithm, a PVI analyser, in combination with the ECGenius system, CathVision's...
An artificial intelligence (AI)-based computer algorithm created by researchers at the Mount Sinai Health System (New York, USA) has been able to learn how to identify subtle changes in electrocardiograms (ECGs) to predict whether a patient was experiencing heart...
Ethnic and racial minority cardiologists report career satisfaction but face professional discrimination and exclusion. This was the main finding of the American College of Cardiology (ACC) professional life survey, published in the Journal of the American College of Cardiology...
Stereotaxis has announced publication of a study in the International Journal of Cardiology: Heart & Vascular demonstrating superior safety and efficacy of Stereotaxis’ robotic technology to treat atrioventricular reentry tachycardia (AVRT) and atrioventricular nodal reentry tachycardia (AVNRT) in paediatric...
Baylis Medical Company has reached an agreement to sell its cardiology business to Boston Scientific.
The acquisition, for an upfront payment of US$1.75 billion, subject to closing adjustments, will see Baylis Medical Company and its cardiology products transition to Boston...
Medtronic has announced the publication of a study demonstrating, through the use of a continuous rhythm monitoring device, that atrial fibrillation (AF) is directly and transiently associated with ischaemic stroke. The findings were published online in JAMA Cardiology.
This retrospective...
Haemonetics Corporation has announced that the Vascade MVP venous vascular closure system is has received a US Food and Drug (FDA) indication for same-day discharge following atrial fibrillation (AF) ablation.
The same-day discharge labelling was granted following the conclusion of...
The US Food and Drug Administration (FDA) has informed healthcare providers of recent information about the potential for differences in procedural outcomes between women and men undergoing implant of a left atrial appendage occlusion (LAAO) device.
The FDA is evaluating...
Abbott has announced that the US Food and Drug Administration (FDA) has approved the company's Amplatzer Talisman PFO Occlusion System to treat people with a patent foramen ovale (PFO)—a small opening between the upper chambers of the heart—who are...
Biosense Webster, part of the Johnson & Johnson Medical Devices Companies has announced post-approval procedures were successfully performed with the Heliostar radiofrequency balloon ablation catheter at sites across Europe. In Europe, the Heliostar Balloon Catheter is indicated for use...
Benjamin D’Souza, assistant professor of Medicine at the University of Pennsylvania, cardiac electrophysiologist at Penn Presbyterian Medical Center, Philadelphia, USA, discusses the recent developments in robotics in the electrophysiology (EP) lab.
The two main criticisms of the use of robotics...
The Heart Rhythm Society (HRS) has announced that its cardiovascular digital health conference—HRX—has been rescheduled to September 2022. The event had been due to take place in San Diego, USA, over 2–4 December 2021.
In a statement issued late last...
Specific and dynamic changes on electrocardiograms (ECGs) of hospitalised patients with COVID-19 or influenza can help predict a timeframe for worsening health and death, according to a new study published in the American Journal of Cardiology. The study, carried...
Continuous heart rhythm monitoring—with anticoagulation if atrial fibrillation (AF) is detected—does not prevent strokes in those at risk. That is the finding of the LOOP study, presented during a Hot Line session at the European Society of Cardiology’s 2021...
Ablation plus cardiac resynchronisation therapy (CRT) is superior to pharmacological rate control in reducing mortality in severely symptomatic permanent atrial fibrillation (AF) patients with a narrow QRS, according to late breaking research presented in a Hot Line session at...
Image-guided fibrosis ablation in addition to pulmonary vein isolation (PVI) does not improve ablation success rates compared to PVI alone in patients with persistent atrial fibrillation (AF), according to an intention-to-treat analysis presented in a Hot Line session at...
The Arrhythmia Alliance’s 2021 Heart Rhythm Congress (A-A HRC 2021 3–6 October, virtual) returns for its second ever digital edition, which will see more than 250 world heart rhythm specialists deliver more than 200 hours of educational content.
In recognition...
Galaxy Medical announced today the initiation of the SPACE-AF study with enrolment of the first two patients at Southlake Regional Health Centre in Newmarket, Canada.
In the study, the Centauri pulsed electric field (PEF) ablation system will be used to...
QardioDirect, the all-inclusive service for remote patient monitoring (RPM) from Qardio, now includes ambulatory cardiac monitoring to help diagnose atrial fibrillation (AF) along with a range of other arrhythmias.
Multiple short duration recordings can identify symptomatic and asymptomatic periods of...
VitalConnect has announced the launch of a new version of VistaCenter for cardiac and remote patient monitoring. This new version brings updates including simplified patient intake, live patient census views, custom notification profiles and report delivery—all designed to streamline...
Medtronic has announced a pilot programme with Mpirik to address disparities in care associated with the prevention of sudden cardiac arrest (SCA), a condition in which the heart suddenly and unexpectedly stops beating. SCA is caused by a disturbance...
Zoll Medical and Itamar Medical have announced that the two companies signed a definitive agreement under which Zoll Medical will acquire all outstanding ordinary shares of Itamar Medical for a total value of approximately US$538 million.
Itamar Medical is focused...
Biotronik has announced the first observational results of the CERTITUDE registry, documenting real-world clinical outcomes of patients in the USA who use implantable loop recorders (ILR), pacemakers, implantable cardioverter-defibrillators (ICDs), and cardiac resynchronisation therapy (CRT-D) devices that have Biotronik...
Stereotaxis and Shanghai Microport EP Medtech have announced a broad collaboration to advance technology innovation and commercial adoption of robotics in electrophysiology in China.
The agreement brings together MicroPort EP’s commercial and product leadership in China’s electrophysiology market with Stereotaxis’...
Bhargav Vemulapalli, Shreya Srivastava and John Kassotis (New Brunswick, USA) review the potential use of antibiotic-eluting envelopes (ABEs) based on available clinical data, while underscoring the need for standardised guidelines related to antibiotic prophylaxis.
Infections associated with cardiac implantable electronic...
AliveCor has announced the results of what it describes as the largest study to assess the reliability of the detection of atrial fibrillation (AF) through commercially available personal ECG devices, in which its KardiaMobile 6L device was found to...
Cardiomatics, the cloud AI tool that allows any trained medical staff to carry out electrocardiogram (ECG) analysis, has raised US$3.2 million in seed funding led by KAYA, the Central and Eastern European VC which has backed companies including Rohlik,...
Edoxaban is noninferior to warfarin and its analogues for adverse clinical events in patients with atrial fibrillation after transcatheter aortic valve implantation (TAVI), new research has found.
Findings of the ENVISAGE-TAVI AF study, which were published in the New England...
Medtronic has announced new data from the Micra Coverage with Evidence Development (CED) study, which showed the Micra transcatheter pacing system (TPS) was associated with a 38% reduction in reinterventions and a 31% reduction in chronic complications at two...
Biosense Webster, part of the Johnson & Johnson Medical Devices Companies, has announced the presentation of data from the inspIRE clinical trial at the European Society of Cardiology’s 2021 congress (ESC 2021, 27–30 August, virtual).
inspIRE is a prospective, multicentre,...
The European Society of Cardiology (ESC) Guidelines on cardiac pacing and cardiac resynchronisation therapy (CRT) have been published online in European Heart Journal.
The use of pacemakers continues to grow as populations worldwide are living longer. It is estimated that...
Abbott has announced late-breaking data from the Amulet LAA Occluder IDE trial, a multicentre, head-to-head study comparing the company's Amplatzer Amulet left atrial appendage (LAA) occluder with the Watchman (Boston Scientific) device to treat patients with atrial fibrillation (AF)...
Abbott has announced results of the landmark GUIDE-HF clinical trial, a 1,000 patient randomised study designed to assess the benefits of the CardioMEMS HF System in people living with NYHA Class II, III and IV heart failure.
Data adjusted for...
The world’s first feasibility study has found that drones can be used to deliver defibrillators to people with suspected cardiac arrest in the community. The research is presented at the European Society of Cardiology’s 2021 congress (ESC 2021, 27–30...
The European Society of Cardiology (ESC) Guidelines for the diagnosis and treatment of acute and chronic heart failure are published online today in European Heart Journal. This was the first ESC Guideline to include patients as full members of...
A six-month exercise programme helps maintain normal heart rhythm and reduces the severity of symptoms in patients with atrial fibrillation (AF), according to late-breaking research to presented this week at the European Society of Cardiology’s 2021 congress (ESC 2021,...
Ultromics, a developer of AI enabled cardiovascular imaging solutions, has announced that it has raised US$33 million in a Series B funding round. The round was led by the Blue Venture Fund with participation from Optum Ventures, GV, and...
The US Food and Drug Administration (FDA) has granted breakthrough device designation to Abiomed’s Impella ECP expandable percutaneous heart pump. The designation means the FDA will prioritise Impella ECP’s regulatory review processes including design iterations, clinical study protocols and...
CardioNXT has received marketing clearance from the US Food and Drug Administration (FDA) for the platform technology comprised of the iMap 3D Navigation & Mapping system, Activate software, Sensor Enabled Axis Patient Patches, and MultiLink sensor enabled catheter.
The CardioNXT...
Biotronik has announced the results from a new study published in EP Europace this week confirming that heart failure (HF) decompensation can be predicted early when monitored using an algorithm that combines existing remote monitoring trends and baseline risk...
People who work night shifts are at increased risk of developing atrial fibrillation (AF), according to research published in the European Heart Journal.
The study is the first to investigate the links between night shift work and AF, and uses...
Abbott has announced that the US Food and Drug Administration (FDA) has approved the company's Amplatzer Amulet Left Atrial Appendage Occluder to treat people with atrial fibrillation (AF) who are at risk of ischaemic stroke. The device offers immediate...
Acutus Medical has received US Food and Drug Administration (FDA) and CE mark clearance for its suite of software upgrades known collectively as AcQMap 8. The upgrades introduce new mapping algorithms into Acutus’ foundational technology, the AcQMap 3D imaging...
Long-term continuous electrocardiogtraphy (LT-ECG), coupled with oversight by a physician, significantly outperforms algorithm-dependent mobile cardiac telemetry (MCT) in the detection of significant arrhythmias according to the findings of a study presented during a late-breaking trial session at the 2021...
Caption Health has announced that the Centers for Medicare and Medicaid Services (CMS) have approved new technology add-on payments (NTAP) for the Caption Guidance AI-based software platform for Medicare patients receiving in-patient care.
Cases eligible for NTAP will have a...
Women remain underrepresented in cardiovascular clinical trials despite initiatives to ensure broader inclusivity. This is according to two reports, both published in the last week, suggesting that lack of representation may limit availability of treatment data for combating cardiovascular...
The challenge of rapid detection and diagnosis of atrial fibrillation (AF) has been made even harder with the onset of the COVID-19 pandemic, and the resulting disruption to appointments and restrictions of access to healthcare services. Against this backdrop,...
Economic analyses from the WRAP-IT study, sponsored by Medtronic, demonstrate the Tyrx cardiac absorbable antibacterial envelope’s (Tyrx Envelope) cost effectiveness in European markets for patients at increased risk of infections.
This follows the publication of a study in Heart Rhythm...
Rhythm control therapy offers a clinical benefit when initiated within one year of a diagnosis of atrial fibrillation (AF) in patients with signs or symptoms of heart failure, a sub-analysis of the EAST–AFNET 4 trial has concluded.
The findings were...
A new analysis of the CABANA (Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation) suggests that catheter ablation offers positive economic outcomes compared to drug therapy for the treatment of atrial fibrillation (AF). This is according to the...
Results of the PAUSE-SCD trial show a significant reduction in risk of ventricular tachycardia (VT) recurrence, cardiovascular hospitalisation, and death when catheter ablation is used as an early first-line treatment for patients with structural heart disease.
The PAUSE-SCD trial is...
Use of direct oral anticoagulants (DOACs) in patients undergoing ventricular tachycardia radio frequency ablation (RFA) is associated with a reduced risk of transient ischaemic attack or stroke, and asymptomatic, magnetic resonance imaging (MRI) detected cerebrovascular event.
This is according to...
Abbott has announced the US launch of Jot Dx, the company’s latest insertable cardiac monitor (ICM). In a press release, Abbott said that the Jot Dx ICM gives clinicians and hospitals control of how they manage the flow of...
Medtronic has announced US Food and Drug Administration (FDA) clearance for two AccuRhythm AI algorithms for use with the LINQ II insertable cardiac monitor (ICM). The algorithms address the two most common ICM false alerts—atrial fibrillation (AF) and asystole.
AccuRhythm...
AliveCor and Acutus Medical will collaborate to assess the integration of data collection tools across the cardiology continuum of care, which the companies say may help guide decision making in the management of cardiac arrhythmia patients.
The collaboration includes a...
Angel Medical Systems (AngelMed) has announced the first commercial implantation and US launch of its The Guardian device. The procedure marks the Guardian's first use following its recent US Food and Drug Administration (FDA) approval. Indicated for acute coronary...
Affera has announced the first-in-human use of its circumferential pulmonary vein isolation (PVI) pulsed field ablation (PFA) catheter, SpherePVI.
The work was conducted as part of a multicentre first-in-human study designed to evaluate the safety and effectiveness of the SpherePVI...
MicroPort Cardiac Rhythm Management (CRM) has entered into definitive agreements in connection with its Series C financing with total investment proceeds of US$150 million.
Hillhouse Capital Group and MicroPort will co-lead the Series C investment and will invest US$20 million...
Medtronic has announced new data from the WRAP-IT study published in Heart Rhythm, which it said demonstrate a significantly lower infection risk for patients who develop haematomas after cardiac implantable electronic devices (CIEDs) when the Tyrx absorbable antibacterial envelope...
People who made even small increases in their daily physical activity levels after receiving an implantable cardioverter defibrillator (ICD) experienced fewer incidences of hospitalisation and had a decreased risk of death, according to research published in Circulation: Cardiovascular Quality...
There is no evidence that moderate coffee consumption can cause cardiac arrhythmia, according to the authors of a prospective, population-based community cohort study, findings of which were published this week in JAMA Internal Medicine.
According to authors Eun-jeong Kim (University...
Two-year results from the PINNACLE FLX clinical trial assessing the safety and efficacy of the next-generation Watchman FLX (Boston Scientific) left atrial appendage closure (LAAC) device for patients with non-valvular atrial fibrillation (NVAF) have shown that patients implanted with...
Ancora Heart has closed US$80 million in equity financing, with the funds to be used to accelerate the CORCINCH-HF pivotal clinical study of the AccuCinch ventricular restoration system. The device is designed to treat patients who have symptomatic heart...
The authors of research published in JACC: Clinical Electrophysiology claim that their findings will help physicians to identify, treat and prevent atrial fibrillation (AF) in patients with hypertrophic cardiomyopathy (HCM).
The research, led by Christopher Kramer the chief of the...
atHeart Medical has announced the successful treatment of the first five patients in its ASCENT ASD US Investigational Device Exemption (IDE) pivotal trial. This study, the company's first in the USA, will evaluate the safety and efficacy of the...
AliveCor has today announced the receipt of 510(k) clearance from the US Food and Drug Administration (FDA) for healthcare professionals to use the KardiaMobile 6L device to calculate patients' QTc interval. This FDA action follows the enforcement policy for...
East End Medical has announced it has received US Food and Drug Administration (FDA) clearance for the company's SafeCross transseptal radiofrequency (RF) puncture & steerable balloon introducer system. The three-in-one system, which includes a steerable introducer sheath with an...
Among racial or ethnic minority patients enrolled in the North American cohort of the CABANA (Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation) trial, catheter ablation significantly improved major clinical outcomes compared with drug therapy. This is according...
Fred Kusumoto (Jacksonville, USA), current president of the Heart Rhythm Society (HRS), sits down with Cardiac Rhythm News to discuss the upcoming Heart Rhythm 2021 meeting which, for the first time, will be a hybrid event consisting of both...
Adagio Medical has announced successful first-in-human use of ultra-low temperature cryoablation (ULTC) for the treatment of monomorphic ventricular tachycardia (VT).
The two-hour mapping and ablation procedure was performed by Tom De Potter, associate director, Cardiovascular Center Department of Cardiology, Electrophysiology...
Royal Philips has announced the first procedure using its real-time 3D intracardiac echocardiography (ICE) catheter. The left atrial appendage occlusion (LAAO) procedure was carried out by Mohamad Adnan Alkhouli (Mayo Clinic, Minnesota, USA).
Used with Philips Premium Cardiology Ultrasound...
The risk of heart damage from COVID-19 infection is far greater than that from receiving a dose of the messenger RNA (mRNA) vaccine, according to the authors of a retrospective case series published in JAMA Cardiology.
Researchers from Mayo Clinic...
Boston Scientific has exercised its option to acquire the remaining shares of Farapulse. The acquisition will complement the existing Boston Scientific electrophysiology portfolio to include the Farapulse Pulsed Field Ablation (PFA) system—a non-thermal ablation system for the treatment of...
Medtronic has announced the initiation of DEFINE AFib, an app-based research study which aims to address unanswered questions around atrial fibrillation (AF) burden and its impact on patient outcomes, quality of life, and healthcare utilisation. The study will use...
Angel Medical Systems (AngelMed) has announced US Food and Drug Administration (FDA) approval of the second-generation AngelMed Guardian device. The AngelMed Guardian system is an implantable cardiac detection monitor and patient-warning system for acute coronary syndrome (ACS) events, including...
The American Heart Association (AHA) has this week issued a scientific statement on the management of atrial fibrillation (AF) in patients with heart failure and reduced ejection fraction (HFrEF).
Published in Circulation: Arrhythmia and Electrophysiology, the statement reviews available evidence...
CardioFocus has announced that the Japanese Ministry of Health, Labor and Welfare (MHLW) has approved the company's HeartLight X3 System for marketing in Japan. The HeartLight X3 System is CardioFocus' catheter ablation technology for controlled and consistent pulmonary vein...
Medtronic has received US Food and Drug Administration (FDA) expanded approval for the Arctic Front family of cardiac cryoablation catheters for the treatment of recurrent symptomatic paroxysmal atrial fibrillation (AF) as an alternative to antiarrhythmic drug (AAD) therapy as...
The Heart Rhythm Society (HRS) has announced the appointment of Simintha Esson as its new chief strategy officer (CSO).
In this role, Esson is responsible for developing and implementing strategies to support the organisation’s short- and long-term business development initiatives....
The president of the European Society of Cardiology (ESC) has called for greater awareness of the signs of cardiac arrest and how to respond, following the collapse of Danish footballer Christian Eriksen, during a European Championship match this week.
Eriksen,...
Imperial College Healthcare NHS Trust (London, UK) is working with remote healthcare developer, Luscii, to provide an at-home monitoring platform to support patients with heart failure.
In its initial phase, the Luscii platform has been rolled out to a distinct...
MicroPort CRM has announced the European launch of Alizea and Borea pacemakers after receiving the CE mark under the new Medical Device Regulation. The devices are equipped with Bluetooth technology for streamlined remote monitoring when paired with the SmartView...
Robert Kowal is the chief medical officer of the Cardiac Rhythm businesses at Medtronic. In this interview he shares his views and experiences on the role that remote monitoring has taken in patients’ lives, both in the past and...
A campaign by Arrhythmia Alliance to DETECT atrial fibrillation (AF)—the most common type of arrhythmia—found that 6% of people aged 65 or older attending a COVID-19 vaccination centre had “possible AF”. The result supports previous findings that the incidence...
Among patients with both heart failure and atrial fibrillation (AF), treatment strategies focused on rhythm control, using catheter ablation, and those focused on rate control, using drugs and or a pacemaker, showed no significant differences in terms of death...
A new physiological measurement of heart function could improve survival for people with heart failure by identifying high-risk patients who require tailored treatments, a study published in the journal Heart, Lung and Circulation suggests.
The study is the first to...
GE Healthcare is collaborating with the American College of Cardiology (ACC) through support of and participation in the ACC’s Applied Health Innovation Consortium for the purpose of building a roadmap for Artificial Intelligence (AI) and digital technology in cardiology...
Medtronic has announced clinical trial results from the STROKE AF trial demonstrating the superiority of the Reveal LINQ insertable cardiac monitor (ICM) to detect abnormal heartbeats, otherwise known as atrial fibrillation (AF), in both large and small vessel stroke...
Abbott has announced it has received CE mark and Health Canada approval for its Amplatzer steerable delivery sheath, which is used with the company's Amplatzer Amulet left atrial appendage (LAA) occluder. Now available in Europe and Canada, the device...
Medtronic is stopping the distribution and sale of the Medtronic HVAD system. This morning, the company notified physicians to cease new implants of the HVAD System and transition to an alternative means of durable mechanical circulatory support.
Medtronic also announced...
Siemens Healthineers has announced that it has received CE Mark for the AcuNav Volume ICE (intracardiac echocardiography) catheter, an imaging guide that provides real-time, wide-angle visualisation of heart anatomy during structural heart and electrophysiology procedures.
“The AcuNav Volume ICE catheter...
Abiomed has acquired preCARDIA, developer of a proprietary catheter and controller to expand options for patients with acute decompensated heart failure (ADHF). The preCARDIA system is designed to rapidly treat ADHF-related volume overload by reducing cardiac filling pressures and...
Implicity has announced a distribution agreement with MicroPort CRM, through which MicroPort CRM can sell and distribute Implicity's cloud-based solution in the USA as part of its remote patient monitoring offerings with a new digital service that allows cardiologists...
Physical activity that conforms to medical and health association guidelines is associated with a lower risk of atrial fibrillation (AF) and stroke, according to a study by analysing the activity of nearly 100,000 individuals equipped with wrist-worn accelerometers to...
The European Union (EU) Medical Devices Regulation (MDR) takes effect from today (26 May 2021).
The Regulation revises quality and safety standards and the range of regulated devices and was first initiated in May 2017, with an initial three-year transition...
iRhythm Technologies has announced two US Food and Drug Administration (FDA) 510K clearances—one for a new and improved design of its flagship monitor and a second for updated artificial intelligence (AI) capabilities.
The new Zio monitor is designed to significantly...
Cardiomatics and the Medical University of Warsaw, Warsaw, Poland are developing a tool for automatic assessment, analysis, and interpretation of electrocardiographic signals (ECG) from paediatric patients.
The Cardiomatics Junior Project is a direct response to the identified lack of ECG...
The US Food and Drug Administration (FDA) has issued advice to patients with implanted devices such as pacemakers or implantable defibrillators to take precautions against the risk of magnetic interference from smartphones and smart watches.
According to the FDA, some...
The proportion of cardiologists reporting burnout has nearly doubled during the COVID-19 pandemic, according to research presented at the American College of Cardiology’s 70th Annual Scientific Session (ACC.21, 15–17 May, virtual). This was among the key findings of the...
Transcatheter left atrial appendage occlusion (LAAO) with a Watchman (Boston Scientific) device was associated with a low rate of stroke at one year, even among older patients with atrial fibrillation (AF) who faced a high risk for stroke or...
Patients with an elevated risk of stroke due to arrhythmia or atrial fibrillation (AF), were much less likely to suffer a stroke after undergoing heart surgery if doctors concurrently performed left atrial appendage occlusion (LAAO), according to the results...
An electrocardiogram (EKG)-based artificial intelligence (AI) algorithm based can enable the early diagnosis of low ejection fraction in patients in routine primary care, according to research published in Nature Medicine.
The ECG AI-Guided Screening for Low Ejection Fraction—EAGLE—trial set out...
Pulmonary vein isolation (PVI) with a “single-shot” pulsed-field ablation (PFA) catheter results in excellent PVI durability and acceptable safety with a low one-year rate of atrial arrhythmia recurrence in paroxysmal atrial fibrillation (AF) patients, according to data pooled from...
Acutus Medical has announced CE mark approval for a broad suite of electrophysiology (EP) products including the AcQCross family of universal transseptal crossing devices, the next generation AcQGuide MAX and VUE large bore delivery sheaths and the next generation...
Discrimination and harassment are a common experience among those working in cardiology, according to the findings from a global survey carried out by the American College of Cardiology (ACC), which have been published in the Journal of the American...
Galaxy Medical (Galaxy) and Japan Lifeline (JLL) have announced an exclusive distribution agreement for the ALPHA1 ablation catheter in the USA, developed by Japan Lifeline for use with the Galaxy Centauri pulsed electric field (PEF) system. Under the terms...
A significant proportion of cardiology trainees are uncomfortable with using telemedicine and feel that better preparation for new-tech medicine is needed, findings of a survey published in the Canadian Journal of Cardiology indicate.
The use of telemedicine has become routine...
Abbott has announced a new trial focused on improving the treatment for people simultaneously battling both atrial fibrillation (AF) and heart failure.
The TAP-CHF trial (Evaluating the Treatment of Atrial Fibrillation in Preserved Cardiac Function Heart Failure) aims to discover...
Subcutaneous implantable cardioverter defibrillator (S-ICD) devices have demonstrated a cardioversion efficacy rate of 98% over a period of at least five years in the EFFORTLESS study—the results of which were presented in a late-breaking presentation at the 2021 European...
AtriCure has announced US Food and Drug Administration (FDA) approval of the EPi-Sense system to treat patients diagnosed with long-standing persistent atrial fibrillation (AF).
The CONVERGE trial demonstrated superiority in the hybrid AF therapy arm compared to endocardial catheter ablation...
Patients hospitalised with COVID-19 may be at risk of developing heart failure even if they do not have a previous history of heart disease or cardiovascular risk factors, a study published in the Journal of the American College of...
Further efforts should be made to increase participation in atrial fibrillation (AF) screening—particularly in elderly populations, where systematic screening for AF can help to lower the risk of adverse events and reduce comorbidities. This is according to a late-breaking...
Patients receiving an implantable cardioverter defibrillator (ICD) should be regularly screened for anxiety and depression, according to research presented at the European Heart Rhythm Association annual meeting (EHRA 2021, 23–25 April, online).
Study author Susanne Pedersen of Odense University Hospital,...
A smartphone-based electrocardiogram (ECG) screening accurately detected previously unknown atrial fibrillation (AF) in American Indians, and more than half who were diagnosed were younger than the recommended screening age of 65, according to new research published in the Journal...
Atrial fibrillation (AF) could be detected during annual foot assessments in patients with diabetes, according to research presented today at the European Heart Rhythm Association annual meeting (EHRA 2021, 23–25 April, online).
The finding was presented by Ilias Kanellos of...
This article was sponsored by Biosense Webster
Putting the patient at the centre of the discussion is vital to setting the right strategy for the treatment of atrial fibrillation (AF). This is according to Biosense Webster’s recently published report: Atrial...
A project at Oxford University Hospitals NHS Foundation Trust (Oxford, UK) to test how effective smart devices are at detecting heart rhythm problems has received a grant of almost £150,000 from national charity Heart Research UK.
Atrial fibrillation (AF) is...
Acutus Medical has announced US Food and Drug Administration (FDA) clearance of the AcQCross family of universal transseptal crossing devices. The transseptal puncture system is specifically engineered to pair with Acutus’ own suite of sheaths and with sheaths sold...
Cardialen has announced the publication of a first-in-human study in the Journal of the American College of Cardiology: Clinical Electrophysiology investigating its low-voltage MultiPulse therapy to terminate atrial fibrillation (AF). The study consisted of 42 patients who were indicated...
The charity Arrhythmia Alliance has launched the Arrhythmia Alliance Healthcare Pioneers Report—Showcasing Best Practice in SVT to provide examples of good care to improve outcomes and quality of life of people with supraventricular tachycardia (SVT).
SVT is a rapid increase...
More patients, particularly those with medical risk factors or from underserved communities, opted into telehealth appointments for their cardiovascular care during the COVID-19 pandemic, a cross-sectional study of more than 176,000 ambulatory cardiology visits in Los Angeles County, USA,...
AF Association has launched an opportunistic screening programme to DETECT atrial fibrillation (AF) at COVID-19 vaccination clinics across the UK. The charity has created an online resource hub for healthcare professionals working at vaccine clinics to use to detect...
The American College of Cardiology (ACC) has launched the EP Device Implant Registry, which will include data on ICD and CRT-D procedures previously captured in the NCDR ICD Registry as well as provide the flexibility to capture novel pacemaker...
Acutus Medical has announced initial US enrolment in the company’s AcQForce Flutter Investigational Device Exemption (IDE) clinical trial. The trial is expected to enrol up to 150 subjects in centres globally and will evaluate the safety and efficacy of...
Dipti Itchhaporia has today begun her term as president of the American College of Cardiology (ACC). During her one-year presidency, she will lead the over 54,000-member global organization in its mission to transform cardiovascular care and improve heart health.
Itchhaporia...
Patients with valvular atrial fibrillation (AF) who were new users of direct oral anticoagulants (DOACs) were at lower risk for ischaemic stroke or systemic embolism and major bleeding than new users of warfarin, an analysis published in the Annals...
More than a third of female cardiologists polled in a UK survey on discrimination and sexism in the profession have reported receiving unwanted sexual comments, attention or advances from a colleague. The findings of the survey were published online...
Tempus has announced that the US Food & Drug Administration (FDA) has granted the company Breakthrough Device Designation for its ECG analysis platform. The platform, developed in collaboration with Geisinger, aids clinicians in identifying patients at increased risk of...
Daiichi Sankyo has announced that it has entered into a collaborative agreement with three technology partners—FibriCheck, neoHealthTech and Capitol Medicare—to improve timely detection and diagnosis of atrial fibrillation (AF).
The partners will be working in collaboration to offer digital solutions...
Bristol Myers Squibb has announced that the US Food and Drug Administration (FDA) has accepted its new drug application (NDA) for mavacamten, an investigational, novel, oral, allosteric modulator of cardiac myosin, for patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM)....
Higher procedural volume is associated with better outcomes for percutaneous left atrial appendage occlusion (LAAO) procedures, research published online in JACC: Cardiovascular Interventions has found.
Outlining the rationale for the study, lead author Salik Nazir (University of Toledo Medical Center,...
An assessment method known as the index-beat approach could improve the diagnosis of heart failure in patients with atrial fibrillation (AF), authors of a study published online in BMJ: Heart have found.
Karina Bunting (Institute of Cardiovascular Sciences, University of...
Medtronic has announced CE mark approval for the SelectSite C304-HIS deflectable catheter system, as well as CE mark labelling expansion for the SelectSecure MRI SureScan Model 3830 cardiac pacing lead for use in His-bundle pacing procedures.
Permanent His-bundle pacing is...
Far from being the first event in the electrophysiology calendar now to make the switch to a digital-only format, the 2021 edition of the Atrial Fibrillation Symposium (AFIB2021, 18 March, virtual) will be the latest to make its digital...
Qardio has announced that the US Food and Drug Administration (FDA) has granted the company 510(k) clearance for its QardioCore ambulatory ECG device. QardioCore will initially be marketed for holter monitoring applications, for use with QardioMD, Qardio's cloud-based remote...
Five-year follow-up results from the Gore REDUCE study, assessing the Gore Cardioform or Helex septal occluder versus antiplatelet therapy in long-term recurrent stroke prevention, have been published in the March 2021 issue of The New England Journal of Medicine (NEJM). The results...
The American College of Cardiology (ACC) has announced a number of the late-breaking clinical trials to be presented at its 70th annual scientific session (ACC.21, 15–17 May, virtual).
The event has recently been switched to an all-virtual format having originally...
CorSystem has announced the first use of its Bipolar Ablation Adapter since the device received CE mark in July 2020.
The patient who underwent treatment was suffering from a VT storm which required frequent interventions of implantable cardioverter-defibrillator. The patient...
Inflammatory heart disease is a rare finding among professional athletes with mild or asymptomatic COVID-19 infection, a large-scale study has found. The study, led by Columbia University Vagelos College of Physicians and Surgeons (New York, USA) in collaboration with...
ControlRad has announced US Food and Drug Administration (FDA) 510(k) clearance to market ControlRad Select, a technology that utilises proprietary semi-transparent filters, a user-interface tablet, and image processing algorithms to reduce radiation exposure during fluoroscopically guided procedures.
The technology is...
Medtronic has announced the first procedures in the investigational device exemption (IDE) pivotal trial to evaluate the PulseSelect pulsed field ablation (PFA) system, a novel, breakthrough technology that uses pulsed electric fields to treat atrial fibrillation (AF).
The first procedure...
Atrial fibrillation (AF) burden at six months after catheter ablation is predictive of all-cause mortality and heart failure hospitalisation, in AF patients with heart failure and low ejection fraction, according to the findings of a subanalysis of the CASTLE-AF...
A global collaboration between international heart failure bodies seeks to standardise the language and practices around the definition and classification of heart failure around the globe.
The Heart Failure Society of America (HFSA), the Heart Failure Association of the European...
An open-source platform, OpenEP, has been made available to advance research on atrial fibrillation (AF), which developers claim can perform 90% of the analyses performed in contemporary electrophysiology studies. The platform, which is detailed in a study recently published...
FARAPULSE has announced that the first patients have been treated in the ADVENT trial, a US Food and Drug Administration (FDA) Investigational Device Exemption (IDE) pivotal trial to evaluate the safety and effectiveness of its pulsed field ablation (PFA)...
iRhythm Technologies has announced the results of the SCREEN-AF study, led by researchers at Sunnybrook Health Sciences Centre in Toronto, Canada and University Hospital in Leipzig, Germany, published in JAMA Cardiology.
The transatlantic clinical trial found that the use of...
Medtronic has announced new results from the REVERSE trial, evaluating outcomes of cardiac resynchronisation therapy (CRT) for patients with mild heart failure (HF). The analysis of the REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) clinical trial shows...
CardioFocus has announced that results from the HeartLight X3 Pivotal Study have been published online in the journal Circulation: Arrhythmia and Electrophysiology (CircEP), showing that all endpoints of the pivotal study were achieved.
The results demonstrated significantly faster pulmonary vein...
Piccolo Medical has announced that it has received US Food and Drug Administration (FDA) clearance of its SmartPICC system.
The SmartPICC system offers the ability to navigate peripherally inserted central catheters (PICCs) to the entryway of the heart without the...
The American College of Cardiology (ACC) has announced that its 70th annual scientific session, will be held as an all virtual meeting. The meeting had been due to be held as a hybrid event, across 15–17 May, with in-person...
Urgent action is needed to address the growing burden of heart failure and the impact of COVID-19 in the UK, according to a report issued this week by the Alliance for Heart Failure.
The report—‘Heart Failure: A call to action’—makes...
RhythMedix has announced the release of its next-generation RhythmStar wearable device with built-in 4G cellular connectivity. The novel cardiac telemetry monitor is worn for extended remote monitoring without the need for an additional phone or communication device.
The technology enables...
MicroPort CRM has launched an online education website—MicroPort Academy CRM—dedicated to cardiac pacing therapy and the functionality of MicroPort CRM products.
MicroPort Academy CRM provides personalised and interactive training plans, ranging from diagnosis of ECG tracings to techniques of pacemaker...
The number of patients with heart failure worldwide nearly doubled from 33.5 million in 1990 to 64.3 million in 2017 according to research published in the European Journal of Preventive Cardiology.
The analysis used data from the Global Burden of...
Updated recommendations on the optimal antithrombotic treatment of patients with atrial fibrillation (AF) receiving oral anticoagulants after undergoing percutaneous coronary intervention (PCI) have been published this week in the journal Circulation.
The recommendations are the latest in a series of...
MedLumics has announced that it has closed an €18 million (US$21.7 million) financing round and has appointed Rich Ferrari as Chairman of the Board of Directors.
The company is developing AblaView, an optically-guided real-time ablation catheter system for the treatment...
CardiacSense has received the CE mark to market and sell a medical grade watch which can detect and remotely monitor atrial fibrillation (AF) and heart rate variability (HRV). The indications certified include detection of AF and monitoring of HRV...
Royal Philips has announced that it has completed the acquisition of BioTelemetry, a US provider of remote cardiac diagnostics and monitoring.
The acquisition of BioTelemetry is a strong fit with Philips’ cardiac care portfolio, and its strategy to transform the...
A study published in the European Journal of Preventive Cardiology provides “strong evidence” of a causal relationship between elevated blood pressure and atrial fibrillation (AF). Authors of the study suggest that strict blood pressure control measures could be an...
B-Secur has announced that it has received US Food and Drug Administration (FDA) 510(K) clearance for its HeartKey software library.
B-Secur’s HeartKey is a suite of EKG/ECG algorithms that combine user identification, health and wellness to generate accurate data encrypted...
The merits of catheter ablation as a first-line treatment for atrial fibrillation (AF) were considered during the second day of AF Symposium 2021 (29–31 January, virtual), which brought together key data from recent clinical trials assessing ablative approaches to...
Hybrid ablation, a procedure which involves “attacking” the atrial substrate from the epicardium and endocardium, has been shown to result in significantly higher freedom from arrhythmia compared to conventional catheter ablation, in persistent and long-standing persistent atrial fibrillation (AF)—late-breaking...
Findings of two studies investigating the use of a lattice-tip catheter for the treatment of atrial fibrillation (AF) were presented during a late-breaking trial session at AF Symposium (29–31 January, virtual) by Vivek Reddy (Icahn School of Medicine at...
Researchers using artificial intelligence (AI) have determined that a smartphone-enabled mobile EKG device can accurately determine a patient’s QT. This can be used to identify patients at risk of sudden cardiac death from congenital long QT syndrome (LQTS) or...
Results of a sub-analysis of the CONVERGE clinical trial, presented during a late-breaking trial session at the digital AF Symposium (29–31 January, virtual), suggest that the hybrid Convergent procedure yields superior outcomes to catheter ablation in patients with long-standing...
Medtronic has received US Food and Drug Administration (FDA) approval for the DiamondTemp ablation (DTA) system which treats patients with recurrent, symptomatic paroxysmal atrial fibrillation (AF) and who have been unresponsive to drug therapy. The DiamondTemp system is the...
Galaxy Medical, a developer of pulsed electric field (PEF) technology for the treatment of cardiac arrhythmias, has announced that the proprietary Centauri system will be featured in a recorded case presentation during the AF Symposium (29–31 January, virtual).
Ante Anić,...
CathVision ApS has announced that its novel cardiac electrophysiology system and artificial intelligence platform to guide ablation therapy for heart rhythm disorders, will be featured at the 26th annual international AF Symposium (29–31 January, virtual).
Josef Kautzner, Institute of Cardiology...
Acutus Medical has announced a number presentations relating to its mapping and ablation procedures during 26th Annual International AF Symposium (29–31 January, virtual). The studies demonstrate the beneficial capabilities of guided ablation therapy utilising the company’s entire diagnostic and...
Farapulse announced that it has received the CE mark for its pulsed field ablation (PFA) system for the treatment of paroxysmal atrial fibrillation (AF). The approval will allow the company to commercialise the cardiac PFA system and permits marketing...
RHYTHM AI has announced publication of a clinical outcome study for its STAR (Stochastic Trajectory Analysis of Ranked signals) Mapping system, which is designed to improve outcomes in patients receiving ablation treatment for persistent atrial fibrillation (AF). The article...
Cardialen has been awarded a US$2.8 million National Institutes of Health (NIH) phase II SBIR grant to further the clinical study of low-energy cardioversion of atrial fibrillation (AF) in human subjects in the USA.
The grant was awarded after a...
Kardium has raised US$115 million in a new financing round, which will be used to accelerate commercial growth of the Globe mapping and ablation system for the treatment of atrial fibrillation (AF) across Europe and to conduct a clinical...
Following the announcement of CE mark approval for the Aktiia cuffless blood pressure monitor bracelet, Gregoire Wuerzner (Lausanne University Hospital and University of Lausanne, Switzerland) clinical advisor to Aktiia and President of the Swiss Society of Hypertension, discusses the...
The wealthiest countries in Europe have higher death rates from atrial fibrillation (AF) than the least wealthy and these death rates are increasing more rapidly than incidence rates, according to an analysis published in the European Heart Journal. The...
Aktiia has announced that its automated blood pressure monitoring system has received the CE mark as a Class IIa medical device. With the CE Mark, Aktiia now has access to over 40 countries worldwide.
Aktiia says that the device is...
InCarda Therapeutics has announced dosing of the first US patient in the company’s multinational INSTANT Phase 2 clinical trial of InRhythm (flecainide for inhalation) in patients with recent-onset paroxysmal atrial fibrillation (PAF). The trial is currently being conducted at...
Boston Scientific has entered into a definitive agreement to acquire Preventice Solutions, a privately-held company which offers a full portfolio of mobile cardiac health solutions and services, ranging from ambulatory cardiac monitors—including short and long-term Holter monitors—to cardiac event...
Robert Klempfner, is director of the Israeli Center for Cardiovascular Research, and director of the Cardiovascular Prevention and the Cardiac Rehabilitation Institutes at Sheba Medical Center, Raman Gat, Israel. In this article he discusses the uses of remote technology...
Patient engagement is key to optimising outcomes in the treatment of atrial fibrillation (AF) argues Elana Arbelo (Consultant Cardiac Electrophysiologist and Community Cardiologist, Hospital Clinic Barcelona, Spain). In this article she discusses how to activate patient engagement through innovation...
Consumer wearables offer a great potential for the screening of atrial fibrillation (AF), however two thirds of healthcare professionals admit to concerns over mass consumer-initiated testing for AF.
These are the findings of a survey published in the European Journal...
“I believe, if you zoom out into the future, and you look back, and you ask the question, ‘What was Apple’s greatest contribution to mankind?’ it will be about health.” This was the vision set out by Tim Cook,...
A study of nearly 108,000 people has found that people who regularly drink a modest amount of alcohol are at increased risk of atrial fibrillation (AF). The study, published in the European Heart Journal, found that, compared to drinking...
Volta Medical, a French health technology startup working on artificial intelligence (AI) algorithms to treat cardiac arrythmias, has announced that it has successfully raised €23 million in financing round led by Gilde Healthcare with participation of existing shareholder Pasteur...
The American College of Cardiology (ACC) is partnering with BioIntelliSense, a continuous health monitoring and clinical intelligence company, to advance remote patient monitoring (RPM) programmes for cardiac care. The partnership will see BioIntelliSense provide the use of its BioButton...
A letter published in the journal Heart Rhythm has warned of possible interference to implantable cardioverter defibrillator (ICD) therapy caused by magnets in the iPhone 12.
The letter, authored by Joshua Greenberg and colleagues from the Henry Ford Heart and...
Jian’an Wang (Heart Center, Zhejiang University, Hangzhou, China) has been named the inaugural editor-of-chief of JACC: Asia, a new journal from the American College of Cardiology. The journal will publish original manuscripts and clinical practice guidelines specific to Asian...
Results of a prospective randomised evaluation of using high power during CLOSE-guided pulmonary vein isolation (PVI) using the Thermocool Smarttouch (Biosense Webster) device—the POWER-AF study—have been published online in Circulation: Arrhythmia and Electrophysiology.
Authors Jean-Yves Wielandts (Department of Cardiology, AZ...
Medtronic Canada has received a Health Canada licence for Micra AV, the world's smallest pacemaker with atrioventricular (AV) synchrony. Micra AV is indicated for the treatment of patients with AV block, a condition in which the electrical connection between...
Elimination of distal coronary sinus (CS) to left atrial (LA) connections reduced atrial arrhythmia recurrences compared to standard pulmonary vein (PV) isolation and non-PV trigger ablation in patients undergoing a first atrial fibrillation (AF) ablation procedure findings of a...
The American College of Cardiology (ACC) has issued guidance recommending against triple antithrombotic therapy in patients with atrial fibrillation (AF), due to its increased risk of bleeding.
The 2020 Expert Consensus Decision Pathway (ECDP), published today, aims to fill the...
Royal Philips has agreed a merger agreement with BioTelemetry, a cardiac monitoring company focused on the diagnosis and monitoring of heart rhythm disorders.
Philips will commence a tender offer to acquire all of the issued and outstanding shares of BioTelemetry...
Abbott has announced that the US Food and Drug Administration (FDA) has approved updated labelling for the company's HeartMate 3 heart pump to be used in paediatric patients with advanced refractory left ventricular heart failure. With the updated labelling,...
Biotronik has today announced US Food and Drug Administration (FDA) clearance and availability of the Vital Data Sensor, which identifies body temperature increases potentially associated with fever, in the new Biomonitor IIIm injectable cardiac monitor (ICM), as shown in...
CardioFocus has announced that more than 10,000 patients worldwide have been treated with the HeartLight endoscopic ablation system.
The HeartLight system is a catheter ablation technology for controlled and consistent pulmonary vein isolation (PVI), the gold standard treatment for atrial...
MicroPort Cardio Rhythm Management (CRM) has announced the first enrolment in the Astral-4LV clinical trial to evaluate the safety and efficiency of Axone, a breakthrough quadripolar left ventricular lead. Axone is designed for use in heart failure patients with...
Acutus Medical has announced CE mark approval and the European launch of its integrated family of transseptal crossing products, designed to deliver safe and efficient access to the left atrium. Coupled with their previously received FDA clearance, Acutus now...
Volta Medical has announced that it has obtained US Food and Drug Administration (FDA) clearance for its VX1 AI (artificial intelligence) software. According to Volta, this is the first FDA clearance for an AI based tool in interventional cardiac...
Biotronik has announced CE mark approval for the AlCath Force the gold-tipped force sensing ablation catheter system.
The AlCath Force was designed specifically to show physicians—in real-time—how much contact is being applied to the heart wall during ablation procedures, helping...
Fourteen drinks a week is linked with a higher risk of stroke and embolism in patients with atrial fibrillation (AF), according to research published in EP Europace.
“Our study suggests that atrial fibrillation patients should avoid heavy alcohol consumption to prevent...
Itamar Medical has announced the publication of a study in the December 2020 edition of Nature and Science of Sleep validating the use of its WatchPAT device against the gold standard in-lab polysomnogram (PSG) in the diagnosis of sleep...
Acutus Medical has announced the launch of the AcQBlate force sensing ablation system in Europe after securing CE mark for the company’s AcQBlate FORCE ablation catheter and the Qubic force sensing module (Qubic Force).
The AcQBlate force sensing ablation system,...
Cathy Gates has been named as the new CEO of the American College of Cardiology (ACC), effective immediately. Gates has been serving as interim CEO of the ACC since April.
“We are excited to have Cathy take the helm of...
The use of direct oral anticoagulants (DOACs) did not reduce the rate of hospitalisation, intensive care admission or fatality due to COVID-19, according to the results of a Swedish observational study, published in The Journal of Internal Medicine.
DOACs are...
iRhythm Technologies has announced it is the first technology to pass through a new digital health tech pilot, resulting in a successful recommendation for adoption from the National Institute for Health and Care Excellence (NICE).
iRhythm’s Zio XT Service—an ambulatory...
Rhythm AI has announced publication of a human clinical outcome study for its Stochastic Trajectory Analysis of Ranked signals (STAR) mapping system in Circulation: Arrhythmia and Electrophysiology. STAR mapping is designed to improve outcomes in patients receiving ablation treatment...
Three-year clinical outcomes of the mHealth Screening to Prevent Strokes—mSToPS—study evaluating silent, or previously undiagnosed, atrial fibrillation (AF) in moderate-risk individuals using Zio, the wearable ECG patch (iRhythm Technologies), were presented at the American Heart Association 2020 scientific sessions...
Atrial fibrillation (AF) was detected up to 10 times more frequently in high risk patients recovering from heart surgery who wore a continuous cardiac monitor for a month, compared to patients who had usual follow-up care following their procedure,...
Medtronic has announced the presentation of results of three studies which it says demonstrate that cryoablation is superior to drug therapy for first-line treatment for the prevention of paroxysmal atrial fibrillation (PAF). Two studies—Cryo-FIRST trial and the investigator-initiated EARLY...
InCarda Therapeutics has announced the presentation of positive data from the open-label, dose-escalation Part A portion of the company’s multinational INSTANT Phase 2 clinical trial of InRhythm (flecainide for inhalation) in patients with recent-onset paroxysmal atrial fibrillation (PAF). Data...
The primary results from the RIVER Trial—Rivaroxaban for Valvular Heart disease and Atrial Fibrillation—show that rivaroxaban is comparable to warfarin for patients with bioprosthetic mitral valves. The findings were presented during a late-breaking trial session at the American Heart...
Adagio, developer of the intelligent Continuous Lesion Ablation System (iCLAS), has announced that it has closed a US$42.5 million Series E equity financing. Proceeds from the financing will be used to support the ongoing iCLAS Investigational Device Exemption (IDE)...
Kardium has announced the first clinical use of the Globe Positioning System—a 3D mapping and navigation feature of its flagship product, the Globe Mapping and Ablation System. The Globe System is designed for the treatment of atrial fibrillation (AF)—involving...
Append Medical, developer of the Appligator implant-free left atrial appendage (LAA) occlusion device, today announced completion of its first pre-clinical chronic procedures. The procedures were completed with the research organisation IMMR and pave the way for the first-in-human trial...
AliveCor has announced the appointment of Toby Cosgrove to its board of directors. For more than 13 years, through to 2017, Cosgrove served as president and chief executive officer of Cleveland Clinic where he was responsible for overseeing the...
HeartHero has announced that its automated external defibrillator (AED) device—known as Elliot—has received CE mark approval. In a press release, the company said that its goal for the device to become the lowest-cost, easiest-to-use, and most portable AED on...
Structural racism is a major cause of poor health and premature death from heart disease and stroke, according to a presidential advisory issued today by the American Heart Association (AHA). “Call to Action: Structural Racism as a Fundamental Driver...
Higher fitness levels reduced the risk of developing atrial fibrillation (AF) by 30‒50% in a study of male, African American veterans, according to preliminary research to be presented at the American Heart Association's Scientific Sessions 2020 (13–17 November, virtual).
“Engaging...
Payments from device manufacturers to physicians are likely to have a bearing on device selection in patients receiving an implantable cardioverter defibrillator (ICD) or cardiac resynchronisation therapy-defibrillator (CRT-D), a study published in the Journal of the American Medical Association...
Rates of procedural success and complications were no different between de novo cardiac resynchronisation therapy (CRT) implantations and upgrades, a study published in JACC: Clinical Electrophysiology has concluded. The study compared rates of procedural success and complications between de...
Abbott has announced has announced the receipt of CE mark and approval in Australia for its EnSite X EP System, and is launching the system throughout Europe and Australia.
Abbott sought physician feedback to develop the EnSite X System to...
Researchers have designed a new machine learning-based approach for detecting atrial fibrillation (AF) drivers, small patches of the heart muscle that are hypothesised to cause this most common type of cardiac arrhythmia. This approach may lead to more efficient...
Boston Scientific has initiated the CHAMPION-AF clinical trial to evaluate the safety and efficacy of the Watchman FLX left atrial appendage closure (LAAC) device within a broad population of patients with non-valvular atrial fibrillation (NVAF), including those who are...
The European Society of Cardiology (ESC) has announced that its full portfolio of congresses, teaching courses and exams in 2021—including the ESC Congress 2021—will be held online, due to the continuing COVID-19 pandemic.
A statement issued on behalf of the...
InCarda Therapeutics, has raised US$30 million through the first close of a Series C equity financing. The financing was led by an affiliate of Innoviva and included existing investors Deerfield Management, HealthCap and Morningside Venture. Proceeds from the financing...
Magnetic resonance imaging (MRI) examinations can be performed safely in patients with non-MR compatible cardiac devices, including those who are pacemaker-dependent or have abandoned leads, according to a study published in Radiology: Cardiothoracic Imaging.
Many patients rely on implanted cardiac...
A research programme into understanding the underlying causes of unexplained cardiac arrest has received significant backing from the British Heart Foundation (BHF).
The four-year project, which is a collaboration between researchers from the UK, Germany and the Netherlands, will study...
The Journal of the American College of Cardiology (JACC) has issued a three-part focus seminar on COVID-19 in 2020 to address the complex relationship between COVID-19 and the heart.
The series includes a paper discussing a new COVID-19-related cardiometabolic syndrome...
Conformal Medical has announced positive clinical data from first-in-human studies performed in the USA and Czech Republic, following trials of its left atrial appendage closure (LAAC) technology. Data were presented at TCT Connect 2020 (14 – 18 October, virtual)...
One in five high-risk patients undergoing major non-cardiac surgery will develop one or more heart complications within a year, according to research published today in European Heart Journal – Acute Cardiovascular Care, a journal of the European Society of...
Coala Life has announced the results of a late breaking trial on cryptogenic stroke patients. In the TEASE-study, recently published in the British Medical Journal, the Coala Heart Monitor was used to evaluate 100 patients after cryptogenic stroke. Patients...
Galaxy Medical, a developer of pulsed electric field (PEF) technology, has announced that the first patients in the ECLIPSE-AF study were successfully treated with the proprietary Centauri system by Ante Anić in Split, Croatia. The multicentre trial is designed...
VivaLNK and AMPS have partnered to advance remote patient monitoring (RPM) for clinical trials, combining AMPS’ continuous electrocardiogram (ECG) recording suite with VivaLNK medical wearable sensors to provide real-time and retrospective analysis for continuous ECG datasets in remote environments.
VivaLNK...
Rhythm AI has today announced that it has received approval from the UK Medicines and Healthcare Products Regulatory Agency (MHRA) to proceed with its multicentre ‘ROCSTAR’ clinical trial. This programme will assess the company’s STAR Mapping System as a...
Patients with postoperative atrial fibrillation (AF) after coronary artery bypass graft (CABG) surgery are at a higher risk of cerebrovascular accidents (CVAs) up to 10 years after the procedure, a study published in Circulation has found. The authors of...
People with atrial fibrillation (AF) have a reduced risk of dementia if they undergo catheter ablation to restore the normal rhythm of their heart, according to a new study published in the European Heart Journal.
Previous work, published last year...
Johnson & Johnson Medical Devices Companies has announced the US Food and Drug Administration (FDA) approval of Biosense Webster’s Thermocool Smarttouch SF ablation catheter for the treatment of persistent atrial fibrillation (AF).
The approval is based on results of a...
AngelMed has announced the first implant of the next-generation AngelMed Guardian system as part of the ALERTS-Continued Access Study. The AngelMed Guardian is an implantable, patient alerting system designed to warn patients to seek medical attention for acute coronary...
SentiAR has received US Food and Drug Administration (FDA) 510(k) clearance for its CommandEP system, the first holographic guidance system to be used during an invasive cardiac procedure. The CommandEP system allows electrophysiologists to visualise 3D electroanatomic models in...
Johnson & Johnson has announced that Biosense Webster has enrolled and treated the first patients in its inspIRE clinical study in Europe. The study will evaluate the safety and effectiveness of the Varipulse catheter and Trupulse generator, investigational technologies...
Shouvik Haldar (London, UK) has been announced as the new A-A Heart Rhythm Congress programme director, following the decision by Kim Rajappan (Oxford, UK) to step down following a four-year tenure.
The new appointment was announced by Trudie Lobban, founder...
Research has identified 2,085 excess deaths in England and Wales linked to cardiovascular disease during the peak of the COVID-19 pandemic. On average, that is 17 deaths each day over four months from 2 March to 30 June 2020....
This advertorial has been sponsored by Boston Scientific
Two landmark studies have been published in 2020 following the use of subcutaneous implantable cardioverter defibrillator (S-ICDs) as a primary prevention therapy for sudden cardiac death. Cardiac Rhythm News speaks to the...
Short-term hydroxychloroquine treatment is not associated with lethal heart rhythms in patients with COVID-19 who are risk assessed prior to receiving the drug. That is the finding of research published today in EP Europace, a journal of the European...
Medtronic has announced the first enrolments in the ALLEVIATE-HF clinical trial which will evaluate the ability of its Reveal LINQ insertable cardiac monitor (ICM) in identifying patients at high-risk of worsening heart failure. The trial will determine if early...
VivaLNK has announced that the University of California, San Francisco (UCSF) (San Francisco, USA), will be utilising its continuous wearable electrocardiogram (ECG) sensor for a 3,000 subject multi-year study on atrial fibrillation (AF). Spanning up to 10 years, the...
Medtronic has received Breakthrough Device Designation status from the US Food and Drug Administration (FDA) for the Tyrx absorbable antibacterial driveline wrap, which is intended to securely hold a percutaneous driveline in patients receiving a ventricular assist device (VAD).
A...
Boston Scientific has signed an investment agreement with an exclusive option to acquire Farapulse, a privately-held company developing a pulsed field ablation (PFA) system for the treatment of atrial fibrillation (AF) and other cardiac arrhythmias.
The PFA system—comprising a sheath,...
Acutus Medical has announces US Food and Drug Administration (FDA) 510(k) clearance of the second-generation AcQMap 3D imaging and mapping catheter which builds on its predecessor, improving handling and deliverability. The 3D mapping and navigation catheter combines 48 ultrasound...
Yassir Javaid (Northampton, UK) discusses the use of cloud-based technologies and machine learning to improve the treatment and detection of arrhythmias.
What potential is there for cloud-based data analytics and machine-learning to improve the outcomes of patients with atrial fibrillation?
Atrial...
Details of the digital format for the Arrhythmia Alliance’s Heart Rhythm Congress 2020 (27–30 September) have been released, with the event to be hosted on a purpose-built virtual conference centre.
The event will include a combination of recorded and live...
Medtronic has announced new data from the WRAP-IT study, showing the TYRX absorbable antibacterial envelope (TYRX Envelope) is cost effective for patients at increased risk of infections who receive cardiac implantable electronic devices (CIEDs). The analysis, newly published in...
CoreMap has announced the completion of a US$10.5 million Series A financing round led by Qure Ventures. The round included existing investors such as Ronny Ginor of Fifth Focus/HRMG Investments and Orbimed Venture Partners, and new strategic, corporate and...
Sufferers of atrial fibrillation (AF) who are exposed to greater levels of pollution have a 1.2-fold higher risk of stroke than their peers who live with less pollution, according to a JAMA Network Open study.
The study carried out by...
VitalConnect has announced the commencement of the TELESTAR-TAVR clinical study—Deployment of Telemedicine for Symptom Tracking And Decrease Readmission Rate in TAVR Patients)—monitoring patients undergoing transcatheter aortic valve replacement (TAVR) using VitalConnect’s VistaSolution LIVE technology. The trial has a primary...
Healthcare professionals should become more familiar with medications that cause arrhythmias, according to a new scientific statement from the American Heart Association (AHA), published in the Association's journal Circulation.
“Many commonly used medications can cause irregular heartbeats as a side...
Stereotaxis has completed installations of the world’s first Genesis Robotic Magnetic Navigation (RMN) systems to treat heart rhythm disorders at Helsinki University Hospital in Helsinki, Finland and Banner University Medicine Heart Institute in Phoenix, USA.
“The clinical benefits of robotics...
Arrhythmia Alliance and its sister charities will launch the first virtual Patients Day on 27 September, with thousands of heart rhythm patients and healthcare specialists set to be in attendance.
The online event will connect patients and arrhythmia specialists to...
Fitbit has received 510(k) clearance from the US Food and Drug Administration (FDA), as well as Conformité Européenne (CE) marking in the European Union, for its electrocardiogram (ECG) app to assess heart rhythm for atrial fibrillation (AF).
The Fitbit ECG...
Acutus Medical has announced pre-clinical experimental results of a novel diagnostic and therapeutic workflow utilising the AcQMap system along with tissue-selective, non-thermal pulse field ablation (PFA) via a proprietary Acutus ablation catheter. While Acutus has been known for its...
iRhythm Technologies has been named a winner of the UK government’s Artificial Intelligence (AI) in Health and Care Award. The announcement was made by Secretary of State for Health, Matt Hancock at London Tech Week’s Founders Forum HealthTech Summit...
The American College of Cardiology (ACC) has announced that its 70th Annual Scientific Session, ACC.21 taking place in Atlanta, USA, will be held across 15–17 May 2021, two months later than the original March dates.
“With continued uncertainty surrounding the...
Royal Philips has announced the next-generation of its Azurion image-guided therapy platform, designed to improve the quality and efficiency of interventional procedures. The Azurion platform has been used in over two million procedures worldwide since its introduction three years...
A study investigating an educational intervention aimed at increasing the use of oral anticoagulants for stroke prevention among atrial fibrillation (AF) patients, found that the intervention made no statistical difference in the uptake of oral anticoagulation. The findings of...
Cardiologs has announced an exclusive distribution agreement in France with MicroPort CRM, for the reselling of its cloud analysis solution for Holter electrocardiograms (ECGs). MicroPort CRM is a distributor of implementable devices for the treatment of heart rhythm disorders....
Digoxin should be considered as a first-line approach for rate control in patients with permanent atrial fibrillation (AF). This was the conclusion of the RATE-AF (RAte control Therapy Evaluation in permanent Atrial Fibrillation) trial, findings from which were presented...
Atrial fibrillation (AF) is associated with long-term mortality in patients undergoing primary percutaneous coronary intervention (PCI), irrespective of left ventricular (LV) function, a study of the prognostic impact of patients undergoing primary PCI with or without LV dysfunction has...
Daiichi Sankyo Europe has announced one-year results of four sub-analyses from the European and global ETNA-AF (Edoxaban Treatment in routiNe clinical prActice in patients with nonvalvular Atrial Fibrillation) programme, a non-interventional safety study evaluating edoxaban treatment in routine clinical...
Patients with newly diagnosed atrial fibrillation benefit from early rhythm control therapy, according to results of the EAST-AFNET 4 trial presented in a Hot Line session at ESC Congress 2020 (Virtual, 29 August–1 September). The findings were published simultaneously...
Cryoballoon catheter ablation as a first line treatment for paroxysmal atrial fibrillation (AF) was found to be safer and more effective than anti arrhythmic drugs, according to a study presented at the ESC Congress 2020 (Virtual, 29 August–1 September).
Oussama...
Patients with arrhythmia should choose the treatment plan with their health professionals, according to European Society of Cardiology (ESC) guidelines published online today in European Heart Journal, and on the ESC website—coinciding with the ESC Congress 2020 (Virtual, 29...
The Heart Rhythm Society has announced the publication of the inaugural issue of the Cardiovascular Digital Health Journal. The new journal focuses on the emerging field of digital medicine across all areas of cardiology. David McManus (UMass Memorial Medical...
Johnson & Johnson Medical Devices Companies has today announced the launch of Biosense Webster’s Carto 3 system version 7 and the Carto Prime mapping module. This represents the most advanced version of the Carto 3 system, the company’s three-dimensional...
Royal Philips has announced new imaging and workflow enhancements for its Kodex-EPD cardiac imaging and mapping system. The system is used to treat patients with atrial fibrillation (AF) at 40 sites worldwide with over 1,500 patients treated. The latest...
Research presented at the ESC Congress 2020 (Virtual, 29 August–1 September) suggests that, among atrial fibrillation (AF) patients, those who quit smoking were 30% less likely to suffer a stroke than those who continue to smoke.
Previous studies have shown...
Daiichi Sankyo Europe has announced the publication of one-year follow-up results from the ongoing ETNA-AF-Europe (Edoxaban Treatment in routiNe clinical prActice for patients with non-valvular Atrial Fibrillation) registry in the European Heart Journal - Cardiovascular Pharmacotherapy.
Published data highlights Europe-specific,...
Ahead of the 2020 European Society of Cardiology (ESC) Congress, taking place from 29 August–1 September, the event’s scientific chair Marco Roffi (University Hospitals of Geneva, Geneva, Switzerland) offers Cardiovascular News a preview of how the digital event is...
Exposure to bridging therapy independently predicts haemorrhagic complications—without improving functional outcomes—in patients with atrial fibrillation (AF) associated stroke. The findings from this retrospective, registry analysis were presented by Feras Akbik (Emory University, Georgia, USA) during a late-breaking abstract session...
J Peter Weiss (Heart Institute, Banner University of Arizona Medical Center, Phoenix, USA) outlines his experience of the AcQMap (Acutus Medical) 3D imaging and mapping catheter system.
How does the AcQMap 3D Imaging and Mapping system differ from other EP...
Johnson & Johnson Medical Devices has today announced European CE mark approval of Biosense Webster’s QDot Micro radiofrequency (RF) ablation catheter.
According to a press release, the next-generation catheter that has demonstrated the ability to reduce total procedure time. This,...
A study by researchers from the Boston University School of Public Health (BUSPH, Boston, USA) shows a decline in deaths related to atrial fibrillation over the last 45 years. But the study, published in the journal BMJ, finds that...
Physicians at the Texas Cardiac Arrhythmia Institute (TCAI) at St David's Medical Center in Austin and Los Robles Health System in Thousand Oaks, became the first in the USA to implant the Watchman FLX left atrial appendage closure (LAAC)...
Patients undergoing atrial fibrillation (AF) ablation, who are physically fit before the procedure, have a much higher chance of benefiting from the procedure and remaining in normal sinus rhythm, according to a study published in Heart Rhythm. Less fit...
The Journal of the American Heart Association (JAHA) has retracted a paper published in March 2020, which drew criticism due to its conclusions about race and the role of affirmative action within cardiology.
The paper, authored by Norman C Wang, associate professor of medicine at the University of Pittsburgh,...
Conformal Medical, a medical device company focused on reducing stroke in patients with atrial fibrillation (AF), today announced it has secured US$85 million in Series C financing.
The financing syndicate included participation from new investors Fidelity Management & Research Company...
Stereotaxis and Acutus Medical have announced the first integrated cardiac ablation procedure using remote TeleRobotic support. Gery Tomassoni of Baptist Health, Lexington, USA treated the patient utilising integrated Stereotaxis Robotic Magnetic Navigation and Acutus AcQMap systems supported by technical...
Eko has announced a collaboration with AstraZeneca to accelerate the development of digital health tools for the earlier screening of cardiovascular diseases, including heart failure.
Through the collaboration, AstraZeneca and Eko will explore accelerating the development of Eko algorithms,...
Abbott has announced first enrolments in the TactiFlex PAF IDE study to evaluate a new device to treat people suffering from paroxysmal atrial fibrillation (PAF). The study will evaluate the performance of the investigational TactiFlex ablation catheter, sensor enabled...
Adagio Medical, developer of the intelligent Continuous Lesion Ablation System (iCLAS), has announced successful pre-clinical results demonstrating its existing cryoablation catheter is also capable of ablating the tissue through pulsed field ablation (PFA) delivered via the existing array of...
Dhiraj Gupta, consultant cardiologist and electrophysiologist at Liverpool Heart and Chest Hospital, (Liverpool, UK) discusses the current burden of atrial fibrillation (AF) on healthcare services, current treatment strategies, and the prospects for arrhythmia treatment post-COVID-19.
What is the current burden...
The risk of electromagnetic interference to cardiovascular implantable electronic devices (CIEDs) from smartwatches and mobile phones is low—although close proximity between an iPhone 6 and an implanted device can cause telemetry interference. These are the conclusions of Philipp Lacour...
The American College of Cardiology (ACC) is expanding its JACC family of peer-reviewed journals to include JACC: Asia, which will publish original manuscripts focused on Asian populations and by Asian authors. The journal will be the first region-specific cardiovascular...
Details of the first-in-human use of an augmented reality holographic display to aid physician accuracy during cardiac ablation have been published in the Journal of the American College of Cardiology (JACC: Clinical Electrophysiology).
Researchers at Washington University (St Louis, USA)...
Caption Health, a developer of medical artificial intelligence (AI) technology, has received US Food and Drug Administration (FDA) 510(k) clearance for an updated version of Caption Interpretation, which enables clinicians to obtain quick, easy and accurate measurements of cardiac...
Digital health and artificial intelligence (AI) company Eko has been awarded a US$2.7 million Small Business Innovation Research (SBIR) grant by the National Institutes of Health (NIH). The grant will fund the continued collaborative work with Northwestern Medicine Bluhm...
Boston Scientific has received US Food and Drug Administration (FDA) approval for the Watchman FLX left atrial appendage closure (LAAC) device. The device is indicated to reduce the risk of stroke in patients with non-valvular atrial fibrillation (NVAF) who...
LifeSignals Group has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for the LifeSignals ECG Remote Monitoring Patch platform.
The platform is a wireless remote monitoring system intended for use by healthcare professionals for the...
Women taking beta blockers for hypertension with no prior history of cardiovascular disease have a nearly 5% higher risk for heart failure than men when they present to hospital with acute coronary syndrome, according to research published in Hypertension,...
The American Heart Association (AHA) has announced that its 2020 Scientific Sessions will be presented as a 100% virtual experience in light of the impact of the COVID-19 pandemic. The event will be held November 13‒17, with registration opening in...
The American College of Cardiology has published an expert consensus decision pathway (ECDP) on the management of bleeding in patients being treated with direct oral anticoagulants (OACs) and vitamin K antagonists for any indication.
Published in the Journal of the...
Screening patients in a primary care setting over one year after occurrence of a stroke using seven-day Holter monitoring uncovered atrial fibrillation (AF) in 4.6% of patients without known AF, a study of the diagnostic benefit of continuous electrocardiogram (cECG)...
Stereotaxis has announced the publication of a paper in the European Heart Journal highlighting the benefits of using robotic technology for the management of ventricular tachycardia storm in a COVID-19 patient. The publication concludes that catheter ablation “should be...
Patients with atrial fibrillation (AF) episodes limited to less than 24 continuous hours had a significantly lower incidence of arrhythmia recurrence following AF ablation according to a study published in JAMA: Network Open. According to the study’s author Jason...
Athena Poppas is chief of cardiology at the Alpert Medical School of Brown University (Providence, USA) and has recently begun her term as the president of the American College of Cardiology (ACC). She tells Cardiac Rhythm News about her...
Jens Erik Nielsen-Kudsk (Aarhus, Denmark) speaks to Cardiovascular News about a propensity score-matched study which examined left atrial appendage occlusion (LAAO) compared with novel oral anticoagulants (NOACs) in atrial fibrillation (AF) patients with a high risk of stroke and...
AliveCor has announced the launch of KardiaCare, a digital health subscription service offering digital tools to monitor heart data, risk factors, identify symptom triggers, and measure the impact of positive lifestyle changes as part of a long-term heart care...
Medtronic today announced that it has received US Food and Drug Administration (FDA) clearance and CE mark approval for its LINQ II insertable cardiac monitor (ICM) device with remote programming. According to a press release from Medtronic, the LINQ...
Abbott has announced that the US Food and Drug Administration (FDA) has approved the company's next-generation Gallant implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) devices.
The new devices offer Bluetooth technology and a new patient smartphone app...
The American Heart Association (AHA) has named Mitchell SV Elkind as president of the organisation for its 2020–2021 fiscal year, which began on 1 July.
In a press release, the AHA says that Elkind is a professor of neurology and...
NuVera Medical has announced the initiation of the company's first-in-human clinical trial to evaluate the performance of its NuVision intracardiac echocardiography (ICE) catheter. The first study participant was successfully treated this month for an atrial septal defect by principal...
inHEART, developer of a cloud-based medical image analysis solution for cardiac interventions on patients with arrhythmias, has closed a funding round of €3.7m led by venture capital firm Elaia. The funds will be used to accelerate commercial development in...
US Food and Drug Administration (FDA) Investigational Device Exemption (IDE) approval has been granted for the Carag Bioresorbable Septal Occluder (CBSO).
The CE-marked CBSO is a transcatheter septal occluder with a bioresorbable, metal-free framework. Carag's first US trial is designed...
Kardium has received CE mark approval for its Globe Mapping and Ablation System for the treatment of atrial fibrillation (AF).
“This is an important milestone both for Kardium and the global AF community,” said Kevin Chaplin, CEO of Kardium. “The...
Left atrial appendage occlusion (LAAO) using the Amplatzer Amulet (Abbott) device has a high implant success rate and a low procedural complication rate in a population at high risk of stroke and major bleeding, one-year outcomes from a roll-in...
Boston Scientific has received US Food and Drug Administration (FDA) 510(k) clearance for the Lux-Dx insertable cardiac monitor (ICM) system, a new, long-term diagnostic device implanted in patients to detect arrhythmias associated with conditions such as atrial fibrillation (AF),...
Left atrial appendage occlusion (LAAO) improves clinical outcomes including ischaemic stroke, major bleeding and all-cause mortality, in atrial fibrillation (AF) patients with a high risk of stroke and major bleeding, compared to the use of novel oral anticoagulants (NOACs)....
Patients with COVID-19 who were admitted to an intensive care unit were 10 times more likely than other hospitalised COVID-19 patients to suffer cardiac arrest or heart rhythm disorders, according to a new study from researchers in the Perelman...
Royal Philips has announced that the Center for Devices and Radiological Health (CDRH) of the US Food and Drug Administration (FDA) has granted premarket approval (PMA) for the company’s HeartStart FR3 and HeartStart FRx automated external defibrillators (AEDs), and...
Voice analysis by a smartphone app can identify lung congestion in heart failure patients, allowing early intervention before their condition deteriorates, a study presented on HFA Discoveries, a scientific platform of the European Society of Cardiology (ESC) has found.
“Speech...
Proximo Medical has announced a partnership with digital health company Coala Life in select US markets. According to a press release from Proximo Medical, it provides a business acceleration solution to addresses medical device commercialisation challenges among startup and...
Medtronic has received CE mark for Micra AV Transcatheter Pacing System (TPS), the world’s smallest pacemaker with atrioventricular (AV) synchrony.
Micra AV is indicated for the treatment of patients with AV block, a condition in which the electrical signals between...
CardioFocus has announced that the first patients have been treated commercially with the recently-approved HeartLight X3 endoscopic ablation system in the USA. The cardiac ablation technology is designed to treat drug refractory, symptomatic paroxysmal atrial fibrillation (AF), the most...
Biotronik has announced its commitment to giving physicians additional tools to pace in the His-Bundle, coinciding with the launch of its His-Bundle Pacing (HBP) tools in a limited number of centres with a full launch later in 2020. The...
Jeffrey L Turner, Brian Zenger and Benjamin Steinberg (all University of Utah School of Medicine, Salt Lake City), write that social media has become a hub of activity for clinical electrophysiologists (EPs) to exchange clinical and scientific material, the...
As part of this year’s HRS 2020 Science virtual event Douglas L Packer (Mayo Clinic, Rochester, USA) delivered the 2020 Eric N Prystowsky lecture on lessons learned from the CABANA trial.
Packer described the lecture as a discussion on “what...
A joint document has been issued by the Heart Rhythm Society (HRS), the American Heart Association (AHA), and the American College of Cardiology (ACC) to provide guidance for clinicians and institutions on re-establishing safe electrophysiological (EP) care.
Published in Heart...
Global cardiac health societies have collaborated on a consensus statement on the diagnosis of arrhythmias, designed to provide physicians with practical proposals to improve patient care in the field.
The international consensus statement on risk assessment in cardiac arrhythmias was...
Researchers have used an optical mapping system to observe how the malaria drug hydroxychloroquine, which has been touted as a possible treatment for COVID-19, creates disturbances in heart rhythm in an animal model. The research, published in the journal...
Adagio Medical has received CE mark approval for its ultra-low temperature intelligent Continuous Lesion Ablation System (iCLAS) for the endocardial treatment of paroxysmal (PAF) and persistent (PsAF) atrial fibrillation and atrial flutter.
“More than half of all atrial fibrillation (AF)...
The British Heart Foundation (BHF) is calling on the Government and the NHS to urgently address the immediate needs of heart and circulatory patients who have had care postponed during the coronavirus pandemic. The charity made its statement after...
As electrophysiologists worldwide begin the process of restarting elective procedures put on hold due to the COVID-19 pandemic, cardiology departments are braced for a wave of “forgotten” patients whose conditions may have worsened over time.
This was among the...
Abiomed has received investigational device exemption (IDE) from the US Food and Drug Administration (FDA) for an early feasibility study with a first-in-human trial of Impella ECP (expandable cardiac power), a 9 French (Fr) heart pump. It will be...
The American College of Cardiology (ACC) is to host a weekly virtual meeting— the Summer COVID-19 Education Series—to provide the healthcare community with actionable insights and solutions that will address key clinical and operational concerns and challenges during the...
The European Society of Cardiology (ESC) has announced that the HFA Discoveries online event will be running six days of live sessions from 5 to 19 June, looking at prevention and treatment strategies in heart failure. The sessions will...
Boston Scientific has announced the US launch of DirectSense technology, a tool for monitoring the effect of radiofrequency (RF) energy delivery during cardiac ablation procedures. According to a press release, the DirectSense technology, which received US Food and Drug...
Highlights:
Cardiologists discuss the "return to normal" as COVID-19 restrictions ease
Late breaking trials from HRS 2020 Science including: PRAETORIAN, UNTOUCHED and PINNACLE FLX
Profile: Athena Poppas
Malcolm Finlay discusses the growing role of artificial intelligence in the diagnosis...
Bardy Diagnostics has received CE mark certification for the 14-day version of the Carnation Ambulatory Monitor (CAM) patch, the p-wave centric ambulatory cardiac patch monitor and arrhythmia detection device. The 14-day CAM patch provides clinicians greater flexibility tomonitor their...
The 2020 edition of the Heart Rhythm Congress (HRC 2020), which had been scheduled to take place 27–30 September in Birmingham, UK, has been moved to an online platform.
Organisers have taken the decision to host the meeting online, as...
A study into the use of hydroxychloroquine or chloroquine, published in The Lancet, has failed to find a benefit in hospital-outcomes from the use of the drugs for the treatment of COVID-19. The study also associates the drug with...
Play the same piece of music to two people, and their hearts can respond very differently. That is the conclusion of a study presented on EHRA Essentials 4 You, a scientific platform of the European Society of Cardiology (ESC)....
Eko has announced the launch of Eko Telehealth, an artificial intelligence (AI)-powered telehealth platform for virtual cardiac and pulmonary monitoring. Being used by more than 200 health systems today, Eko Telehealth incorporates stethoscope and ECG live-streaming combined with embedded...
Acutus Medical and Biotronik have announced a new alliance to provide a comprehensive portfolio of electrophysiology, mapping, ablation and accessory products for catheter-based treatment of cardiac arrhythmias across select markets, including Europe and Asia.
At the centre of the new...
Cardiologs, in partnership with the Valley Health System, has announced the results of a clinical study that found the Cardiologs AI-based ECG analysis solution reduces by almost 70% the false positives of atrial fibrillation (AF) detected by implantable loop...
Thermedical has announced that results from a first-in-human early feasibility study (EFS) using the Durablate catheter to treat ventricular tachycardia (VT) were presented in the late-breaking clinical trial sessions at the Heart Rhythm Society (HRS) 2020 Science online meeting.
Titled...
The American College of Cardiology (ACC) is to collect COVID-19 data through the NCDR Chest Pain-MI and CathPCI registries with the aim of capturing the relationship between COVID-19 and heart disease and maximise data on the cardiac impact of...
Royal Philips has announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) to market a wide range of its ultrasound solutions for the management of COVID-19-related cardiac and lung complications.
Handheld and portable ultrasound...
David E Albert, founder and chief medical officer of AliveCor, considers the impact of the COVID-19 pandemic on the monitoring and of management of arrhythmias.
As the world continues to fight against the COVID-19 pandemic, healthcare providers and professionals are...
The US Food and Drug Administration (FDA) has expedited clearance of an update to software from Caption Health, a medical artificial intelligence (AI) company, that is designed to aid frontline healthcare workers to perform diagnostic-quality cardiac ultrasounds.
Following a...
CardioFocus has announced that the US Food and Drug Administration (FDA) has approved the next-generation HeartLight X3 endoscopic ablation system for the treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation (PAF).
Approval of the HeartLight X3 system came as...
Medtronic has received US Food and Drug Administration (FDA) approval for its Cobalt and Crome implantable cardioverter-defibrillators (ICD) and cardiac resynchronisation therapy-defibrillators (CRT-D).
ICDs monitor heart rhythms and deliver therapy to correct heart rates that are too fast and can...
Corresponding author Valentin Fuster (Mount Sinai Heart, New York, USA) and colleagues report in the Journal of the American College of Cardiology that hospitalised COVID-19 patients treated with anticoagulants had improved outcomes both in and out of the intensive...
AtriCure has announced results from the CONVERGE IDE clinical trial, which showed an 18% difference in favour of the hybrid Convergent procedure as compared to endocardial catheter ablation in the treatment of atrial fibrillation (AF). The results of the...
Findings from the UNTOUCHED study, presented online in a late-breaking trial session at HRS 2020 Science, showed that patients receiving subcutaneous implantable cardioverter defibrillator (S-ICD) therapy experienced a higher inappropriate shock-free rate than in previous S-ICD and transvenous implantable...
Medtronic has announced results from late breaking clinical trials, presented online at HRS 2020 Science, evaluating the MyCareLink Heart mobile app and the Micra Transcatheter Pacing System (TPS).
Results from the BlueSync Evaluation study demonstrated that patients who used the...
Contact force (CF) sensing catheters have been shown to be safe and effective in persistent atrial fibrillation (AF) ablation, according to the findings of the PRECEPT study, which have been presented online during a late-breaking clinical trial session at...
Boston Scientific has announced positive 12-month results from the PINNACLE FLX clinical trial assessing the safety and efficacy of the next-generation WATCHMAN FLX Left Atrial Appendage Closure (LAAC) Device for patients with non-valvular atrial fibrillation (NVAF). Presented as a...
Subcutaneous implantable cardioverter defibrillators (S-ICDs) are as protective as transvenous implantable cardioverter-defibrillators (ICDs) in the prevention of sudden cardiac death, but have a better safety profile, the results of a prospective, randomised trial online in a late-breaking trial session...
Initial results of the PULSED AF trial, the first-in-human results for paroxysmal or persistent atrial fibrillation (AF) treated with pulsed field ablation (PFA) were presented online as part of a late breaking clinical trial session at Heart Rhythm 2020....
The Heart Rhythm Society (HRS) has released a white paper announcing the rationale and core components for Atrial Fibrillation (AF) centres of excellence (CoE), to provide multidisciplinary care for AF patients. The goal of the white paper is to...
Fitbit has today launched the Fitbit Heart Study, its first large-scale, virtual study to validate the use of its wearable technology to identify episodes of irregular heart rhythm suggestive of atrial fibrillation (AF). This study is part of the...
The European Society of Cardiology (ESC) is for the first time in its 70-year history, to hold its annual meeting (ESC Congress) 100% online. A press release states that the move follows an announcement from the Dutch government banning...
The American College of Cardiology (ACC) and other North American cardiology societies, including the Canadian Association of Interventional Cardiology (CAIC), have issued guidance on ethically and safely reintroducing invasive cardiovascular procedures after the peak of COVID-19 has passed. The...
LifeSignals has received CE mark approval for the LifeSignals ECG Remote Monitoring Patch, a disposable wireless remote monitoring system, intended for use by healthcare professionals for continuous collection of Electrocardiography (ECG) and heart rate monitoring in ambulatory, hospital, healthcare...
Results of a clinical trial show stark disparities in the use of life-saving implantable cardioverter defibrillator (ICD) interventions based on sex and race, suggesting a potential bias in care pathways by electrophysiologists (EPs). The study was presented online as...
VitalConnect has been granted Emergency Use Authorisation (EUA) status by the US Food and Drug Administration (FDA) as part of the response to the COVID-19 pandemic. The EUA will enhance the capabilities of the VitalPatch and continuous patient monitoring...
A necklace that detects abnormal heart rhythm will be showcased for the first time on EHRA Essentials 4 You, a scientific platform of the European Society of Cardiology (ESC).
According to the study's author, Elmeri Santala from the University of...
A necklace that detects abnormal heart rhythm will be showcased for the first time on EHRA Essentials 4 You, a scientific platform of the European Society of Cardiology (ESC).
According to the study's author, Elmeri Santala from the University of...
A study published in the Journal of the American College of Cardiology (JACC) provides the first direct evidence that KCNQ1 antibodies are able to suppress arrhythmias in a cellular model of long QT syndrome type 2 (LQTS2), its authors...
Prism Schneider (Cumming School of Medicine, University of Calgary, Calgary, Canada) and others write in a commentary in the Canadian Medical Association Journal—because of an increase in domestic violence during the pandemic—healthcare providers should be aware of the signs...
Same-day discharge after atrial fibrillation (AF) ablation is feasible for the majority of patients when a standardised protocol is used, findings of a multicentre cohort study, published in the Journal of the American College of Cardiology (JACC), suggest. Efficacy and safety of a same-day discharge protocol were studied by...
On 18 March, the Centers for Medicare & Medicaid Services (CMS) recommended “limiting non-essential care and expanding surge capacity into ambulatory surgical centres and other areas” to conserve resources and staff for managing COVID-19 patients. However, in a statement...
iRhythm Technologies has announced a virtual platform to support patients and clinicians in the wake of COVID-19 by offering home enrolment for the company’s Zio XT and Zio AT heart monitoring devices.
Since patients with heart conditions are a particularly...
Coala Life has announced the expansion of capabilities to help monitor patients from home using the Coala Heart Monitor. This expansion is a result of the US Food and Drug Administration’s (FDA) new Emergency Guidance striving to help battle...
iRhythm Technologies has announced the appointment of Renee Budig to its Board of Directors effective 23 April 2020. Budig was appointed as a Class II director and will also serve on the Audit Committee.
“On behalf of the iRhythm team,...
The US Food and Drug Administration (FDA) has issued a Drug Safety Communication regarding known side effects of hydroxychloroquine and chloroquine, including serious and potentially life-threatening heart rhythm problems, that have been reported with their use for the treatment...
The use of mobile cardiac outpatient telemetry (MCOT) can help monitor COVID-19 patients taking hydroxychloroquine and azithromycin, while reducing potential exposure to the virus for clinicians and preserving personal protective equipment (PPE), according to a study published in the...
The Heart Rhythm Society (HRS) COVID-19 Rapid Response Task Force has updated its guidance on QTc monitoring in COVID-19 patients. In the revised guidance, the society provides recommendations for inpatient management of COVID patients recevieing hydroxychloroquine and advises against...
The European Society of Cardiology (ESC) is participating in two large-scale EU-funded projects to detect heart rhythm disorders and prevent sudden cardiac death.
The first of the projects, AFFECT-EU, aims to develop a universal screening algorithm for atrial fibrillation (AF),...
Cardiologs has announced the launch of a clinical study using the company’s artificial intelligence (AI) solution to remotely monitor the cardiac safety of COVID-19 patients during hydroxychloroquine treatment via analysis of electrocardiogram (ECG) data gathered from smartwatches.
Hydroxychloroquine therapy is...
Cardiac Insight has announced the launch of the Heart@Home electrocardiogram (ECG) test kit to address new telehealth demands placed on cardiology practices by the COVID-19 response.
The kit enables physicians to prescribe the arrhythmia diagnosis technology of the Cardea SOLO...
In response to a recent American College of Cardiology (ACC) review that advocated that troponin should only be measured when a diagnosis of acute myocardial infarction is being considered, Andrew R Chapman (BHF Centre for Cardiovascular Science, Royal Infirmary...
DiNovA Medtech has announced that Vivek Reddy will join the company as chief medical advisor and co-founder of portfolio company Nuomao Medtech to lead cardiac arrhythmia related innovations.
Reddy is the Director of Cardiac Arrhythmia Services for the Mount Sinai...
The COVID-19 pandemic is hitting the world hard. Egypt, the most populous country in the Middle East and North Africa (MENA) region, is no exception. With more than 2,500 confirmed cases and a preliminary case fatality rate of 7%...
The American Heart Association (AHA) has compiled interim CPR guidelines to help rescuers treat cardiac arrest patients with suspected or confirmed COVID-19. The guidelines are in collaboration with the American Academy of Pediatrics, American Society of Anesthesiologists, American Association...
In a brief report in Annals of Internal Medicine, Seongman Bae (Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea) and colleagues report that neither surgical nor cotton masks appear to be an effective approach for preventing the dissemination of SARS-CoV-2 from...
Physicians already recognised the importance of remote management for heart failure patients long before the COVID-19 pandemic. However, the current situation has heightened the urgency of the transition for this vulnerable population. In this commentary, Michael Kiernan reviews how...
Bardy Diagnostics has announced that its Carnation Ambulatory Monitor (CAM) Patch has been chosen for use in protocols to measure QT Segments in COVID-19 patients who have been prescribed hydroxychloroquine (HCQ).
BardyDx’s CAM Patch will be utilised in newly established...
Presidents of leading cardiac organisations, including the American Heart Association (AHA), the American College of Cardiology (ACC) and the Heart Rhythm Society (HRS), have published a statement that cautions against the use of hydroxychloroquine and azithromycin for the treatment...
Royal Philips has announced that the US government and Philips agreed to team up to increase the production of hospital ventilators in its manufacturing sites in the USA.
Philips plans to double the production by May 2020 and achieve...
Medtronic has announced updates regarding its efforts to increase ventilator production around the globe. The company is announcing progress in the ramp-up of its ventilator production, as well as collaborating with technology partners and governments to drive new ventilator...
Medtronic has announced it is publicly sharing the design specifications for the Puritan Bennett 560 (PB 560) to enable participants across industries to evaluate options for rapid ventilator manufacturing to help doctors and patients dealing with COVID-19. This decision...
Cardiovascular specialists from across the globe shared current knowledge and key experience of managing COVID-19 patients as part of a webinar organised by the Imperial College Network of Excellence in Vascular Science at Imperial College London.
The four-part COVID-19 Cardiovascular...
The American Heart Association (AHA), in conjunction with the American Association for Respiratory Care (AARC) and American Society of Anesthesiologists (ASA) is offering guidance in oxygenation and ventilation management for healthcare providers as hospital volumes surge from COVID-19.
AHA released...
The reduced levels of angiotensin converting enzyme (ACE) 2 associated with cardiovascular disease and increasing age lead to a higher susceptibility to greater disease severity in COVID-19. A viewpoint published online in JAMA Cardiology hypothesises that severe acute respiratory...
The European Commission (EC) has adopted a proposal to postpone by one year the date of application of the new Medical Devices Regulation (MDR), which was due to come into force on 26 May this year. The postponement, a press...
The Heart Failure Association (HFA) of the European Society of Cardiology (ESC) has announced that Heart Failure 2020 & the World Congress on Acute Heart Failure, scheduled to take place over 23–26 May in Barcelona, Spain, has been cancelled.
In...
Athena Poppas (Providence, USA) is the new president of the American College of Cardiology (ACC). Her term officially began at the close of the ACC/World Congress of Cardiology’s virtual scientific sessions (ACC.20/WCC Virtual).
Poppas has been a long-standing leader of...
Simon Ray, president of the British Cardiovascular Society (BCS), has told the COVID-19 Cardiovascular Conference that a lack of consistency in national guidance around personal protective equipment (PPE) during COVID-19 had led to a “vacuum” which has had to...
In a new statement published on 2 April 2020, all 45 societies represented by the US Council of Medical Specialty Societies (CMSS)—over 800,000 physicians—emphatically declare their belief that all frontline healthcare professionals must have access to personal protective equipment...
A raised troponin level in myocardial injury “greatly alters the prognosis adversely” in COVID-19 patients, Graham Cole (Imperial College Healthcare NHS Trust, London, UK) told a virtual cardiovascular conference, hosted today by Imperial College London.
Cole explained: “Troponin rises occur...
The COVID-19 Cardiovascular Conference, hosted as a live webinar by The Imperial College Network of Excellence in Vascular Science at Imperial College (London, UK), has heard about experiences from both Italy and the USA, as well as details of...
The Heart Rhythm Society (HRS), The American College of Cardiology (ACC), and the American Heart Association (AHA) have released a joint document dealing with the impact of COVID-19 on electrophysiology.
Published online in Heart Rhythm by Dhanunjaya Lakkireddy (Kansas City...
Due to COVID-19 concerns and travel restrictions, the 2020 Heart Rhythm Scientific Sessions (HRS 2020) will not take place in San Diego, USA, on 6–9 May as planned, the Heart Rhythm Society has announced.
Instead, HRS will offer several new...
Daniel M Philbin Jr has been named chair of the American College of Cardiology (ACC) board of governors and secretary of the board of trustees, the main governing body of the ACC, for 2020–2021. His term began at the...
The Imperial College Network of Excellence in Vascular Science at Imperial College London (UK) is to run a COVID-19 Cardiovascular Conference as a live webinar on Thursday 2 April for all healthcare professionals battling COVID-19.
The webinar aims to share...
Results from the phase 2 MAVERICK-HCM clinical trial, presented at the American College of Cardiology/World Congress of Cardiology’s virtual scientific sessions (ACC.20/WCC Virtual) suggest that the use of mavacamten (MyoKardia) for the treatment of non-obstructive hypertrophic cardiomyopathy (nHCM) may be...
Data on the use of LivaNova’s implantable neuromodulation technology, Vitaria, for heart failure patients has been shared at the American College of Cardiology/World Congress of Cardiology’s virtual scientific sessions (ACC.20/WCC Virtual).
Two posters were published and shared on demand at the...
The infusion of ethanol via the vein of Marshall (VOM), coupled with catheter ablation, leads to improved outcomes for patients with persistent atrial ablation, according to the findings of a study presented by Miguel Valderrabano (Baylor College of Medicine, Houston, USA) at the American College of Cardiology/World Congress of Cardiology’s...
Data from the National Cardiovascular Data Registry (NCDR) Left Atrial Appendage Occlusion (LAAO) Registry, which enrolled more than 38,000 patients implanted percutaneously with the Watchman device (Boston Scientific), reveal a high implant success rate and a low rate of...
During a Q&A section of a European Commission (EC) college meeting on 25 March, EC spokesperson Stefan de Keersmaecker stated that the commission were looking to delay the “entry into force” of the new European medical device regulations (MDR) because of...
A viewpoint in the Journal of the American Medical Association (JAMA) has offered potential solutions to modifying ongoing randomised clinical trials during the COVID-19 pandemic. It aims to “minimise disruption and preserve integrity”, while still ensuring participant health and...
Cardiac injury is common among hospitalised patients with COVID-19, and is associated with a higher risk of in-hospital mortality, a study from Wuhan, China, has shown. Although the exact mechanism of cardiac injury has not been defined, the researchers...
The US Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) has written to the medical device industry to outline its response to the COVID-19 public health emergency in its day-to-day operations with industry.
The letter from...
The American Heart Association (AHA) has committed US$2.5 million to research efforts to better understand the novel coronavirus, COVID-19, and its interaction with the body’s cardiovascular and cerebrovascular systems.
Specifically, the Association will be offering fast-tracked research grants for short-term...
The British Cardiovascular Intervention Society (BCIS) and the British Cardiovascular Society (BCS) have endorsed guidance published by NHS England and NHS Improvement on the provision of cardiology services during the COVID-19 pandemic.
A joint statement from Nick Curzen, president of...
Aktiia, a Swiss-US start-up that has developed an optical blood pressure monitor at the wrist, has raised an additional CHF 6 million in funding to bring its product to market. The technology combines common optical sensors and proprietary clinically...
AliveCor has announced that its KardiaMobile 6L is now allowed for use in the measurement of a patient’s QTc and detection of potentially dangerous QT prolongation. A prolonged QTc can lead to a potentially fatal side effect of drug-induced...
A case series of US experience with COVID-19 has upheld suggestions of a link with cardiomyopathy, which developed in 33% of intensive care patients.
In a research letter published in the Journal of the American Medical Association, Matt Arentz (Department...
The US Food and Drug Administration (FDA) has issued an immediately-in-effect guidance that allows manufacturers of certain FDA-cleared non-invasive, vital-sign-measuring devices to expand their use so that health care providers can use them to monitor patients remotely.
The devices...
The American College of Cardiology Scientific Session Together with World Congress of Cardiology (ACC.20/WCC) is to run as a virtual meeting, following its cancellation due to the coronavirus outbreak. It was scheduled to take place on 28–30 March 2020...
Portola Pharmaceuticals announced new data that they say reinforces the value of Andexxa (coagulation factor Xa , inactivated-zhzo). The company statement outlines that the data demonstrate Andexxa was associated with a lower rate of in-hospital and 30-day mortality in...
Cephalic vein cutdown (CVC) and axillary vein access (AVA) are both effective approaches for cardiac implantable electronic device (CIED) lead implantation, and could avoid the complications usually observed with traditional subclavian vein puncture (SVP). This was the conclusion of...
Researchers have derived several algorithms to identify emergency department (ED) oral anticoagulant prescriptions for patients with atrial fibrillation (AF) in a large health dataset. The retrospective cohort study identified algorithms that, they say, “can be selected to optimise specificity,...
Almost 70% of patients treated with a leadless left ventricular (LV) endocardial pacing system had a favourable clinical response at six months, a post-market surveillance registry has shown.
The findings were published in Heart Rhythm. Authors Benjamin J Sieniewicz (St...
Miguel Angel Cobos Gil (Instituto Cardiovascular, Hospital Clinico San Carlos, Madrid, Spain) outlines in the Annals of Internal Medicine a novel method of using the Apple Watch to record a multi-lead, quasi-standard electrocardiogram (ECG). He reports that the method...
The novel left atrial appendage (LAA) closure device Appligator (Append Medical) was a finalist in the 2019 ICI Innovation Award Competition (Innovation in Cardiovascular Interventions 2019; 8–10 December, Tel Aviv, Israel). Leonid Sternik, who came up with the concept...
Impulse Dynamics, developer of cardiac contractility modulation (CCM) therapy delivered by the Optimizer Smart System, has announced the appointment of Ishu Rao as medical director.
A press release from the company states that Rao is a board-certified cardiac electrophysiologist who...
The scientific congress of the European Heart Rhythm Association (EHRA 2020) and the Scientific Session of the American College of Cardiology Together with World Congress of Cardiology (ACC.20/WCC) have both been cancelled due to the coronavirus outbreak.
EHRA 2020 was...
Stereotaxis has received US Food and Drug Administration (FDA) 510(k) clearance for the Genesis Robotic Magnetic Navigation (RMN) system for the robotic navigation of magnetic ablation catheters to treat heart rhythm disorders.
“Genesis is a leap forward in Robotic Magnetic...
James Teo was the chief investigator in the EPACS clinical trial, testing the performance of the Zio Patch (iRhythm Technologies) wearable biosensor. He outlines to Cardiac Rhythm News his experience with the device and discusses its use in the...
Rapid high-current, temperature-controlled point-by-point pulmonary vein isolation (PVI) and linear ablation has been shown to be clinically feasible and safe in a first-in-human study of an expandable radiofrequency lattice tip catheter. Other findings from the same study, announced last...
Twenty late-breaking trials, including major clinical studies on the treatment of atrial fibrillation (AF) are among the research to be presented at the annual congress of the European Heart Rhythm Association (EHRA) 2020 (29–31 March, Vienna, Austria).
The late-breaking trials...
Kalyanam Shivkumar has been named the next editor-in-chief of the Journal of the American College of Cardiology (JACC): Clinical Electrophysiology.
Shivkumar is a physician scientist who serves as the director of the University of California, Los Angeles (UCLA) Cardiac Arrhythmia...
Wearing an adhesive patch sensor that streams data to an analytical platform can accurately predict worsening heart failure (HF) and the need for hospitalisation several days before it is necessary, according to research published in Circulation: Heart Failure.
The LINK-HF...
Append Medical, developer of the Appligator, a novel left atrial appendage (LAA) closure device to minimise stroke risk in atrial fibrillation (AF) patients, and Sheba Medical Center (Ramat Gan, Israel), have announced the clinical proof-of-concept data evaluating the safety...
Abbott has announced that it has received CE mark approval for the Gallant implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) devices.
The devices offer new opportunities for patient engagement and remote monitoring through new smartphone connectivity and...
A study of patients undergoing implantable cardioverter defibrillator (ICD) therapy across three centres, found that only one in three were treated in concordance with current guidelines on ICD device programming. This is the finding of research led by Teetouch...
VitalConnect has announced the introduction of arrhythmia detection to its remote monitoring portfolio. In partnership with CorVitals, VitalConnect now offers physicians the ability to monitor, diagnose and treat patients suffering from various heart conditions and diseases.
With arrhythmia detection, clinicians...
The American College of Cardiology (ACC) and automated external defibrillator (AED) technology start-up HeartHero have formed an alliance aimed at making a “significant impact” on survival rates after sudden cardiac arrest (SCA).
The partnership will further ACC’s mission to transform...
The US Food and Drug Administration (FDA) has authorised marketing of software to assist in the acquisition of cardiac ultrasound, or echocardiography, images. The software, called Caption Guidance (Caption Health), is an accessory to compatible diagnostic ultrasound systems and...
CardioFocus has submitted a pre-market approval (PMA) supplement to the US Food and Drug Administration (FDA) for the HeartLight X3 Endoscopic Ablation System for the treatment of atrial fibrillation (AF). This supplement follows the original, approved PMA of the...
Jennifer Silva (St Louis, USA) talks to BLearning Cardio at SIR 2019 (Society of Interventional Radiology; 23–28 March; Austin, USA) about why physicians should move away from using Virtual Reality (VR) during procedures and move towards augmented and mixed...
RHYTHM AI has announced the signing of a research collaboration agreement with Biosense Webster. A press release from RHYTHM AI outlines that the agreement grants it access to certain data from the Biosense Webster CARTO 3 System to support...
Abbott has announced approval from the US Food and Drug Administration (FDA) for the CATALYST trial to examine its Amplatzer Amulet device compared to non-vitamin K oral anticoagulants to lower stroke and bleeding risks for patients with atrial fibrillation.
A...
Medtronic has received CE Mark for its Cobalt and Crome portfolio of implantable cardioverter-defibrillators (ICDs) and cardiac resynchronisation therapy-defibrillators (CRT-D). ICDs monitor heart rhythms and deliver therapy to correct heart rates that are too fast and can lead to...
Eko has announced that the US Food and Drug Administration (FDA) has cleared a suite of algorithms that, when combined with Eko’s digital stethoscopes, will enable healthcare providers to more accurately screen for heart conditions during routine physical exams....
Hugh Calkins (Johns Hopkins Medical Institutions, Baltimore, USA) told delegates attending the AF Symposium 2020 (23–25 January, Washington, DC, USA) that cryoablation with the Arctic Front catheter (Medtronic) was a safe and effective approach for managing persistent atrial fibrillation...
At the AF Symposium 2020 (23–25 January, Washington, DC, USA), Vivek Y Reddy (Helmsley Electrophysiology Center, Icahn School of Medicine at Mount Sinai, New York, USA) reported that pulsed field ablation (PFA)—with the Farawave (Farapulse Inc.) catheter—may be a feasible and...
Acutus Medical offered a demonstration of its AcQMap advanced cardiac imaging and mapping system at AF Symposium (23–25 January, Washington, DC, USA) with a live-stream of a case study from Oxford University Hospitals. The live stream, which was followed by...
Speaking today at the AF Symposium (23–25 January, Washington, DC, USA), Francis Marchlinski (Hospital of the University of Pennsylvania, Philadelphia, USA) reported that selected atrial fibrillation (AF) patients could safely and effectively take non-vitamin K antagonist oral anticoagulants (NOACs)...
Medtronic has received approval from the US Food and Drug Administration (FDA) to proceed with an investigational device exemption (IDE) trial to evaluate the safety and effectiveness of the PulseSelect Pulsed Field Ablation (PFA) System, a new technology that...
Bardy Diagnostics (BardyDx) has today announced the commercial launch of the 14-Day version of the Carnation Ambulatory Monitor (CAM), the P-wave centric ambulatory cardiac patch monitor and arrhythmia detection device, following recent clearance by the FDA.
“The 14-Day CAM...
Biotronik has announced the market release of its injectable cardiac monitor (ICM), BioMonitor III in Japan. The device is designed to help patients with irregular heart rhythms by documenting unexplained syncope with increased clarity.
In a press release, Biotronik noted...
A technique that enables patients suffering from heart conditions to hold their breath safely for over five minutes could have potential as a treatment for cardiac arrhythmias, according to research lead by Michael Parkes (University of, and University Hospitals...
Malcom Finlay (Bart's Health Centre, London, UK) reviews the growing role of artificial intelligence (AI) in diagnosing arrhythmias.
Johnny, a 72-year-old film producer, is meeting clients when his watch gently vibrates notifying him of his irregular pulse. He pressed his...
Medtronic has announced that it has received US Food and Drug Administration (FDA) approval of Micra AV, the world’s smallest pacemaker with atrioventricular (AV) synchrony.
Micra AV is indicated for the treatment of patients with AV block, a condition in...
CardioFocus, has announced that the company’s board of directors has promoted Burke T Barrett to the position of chief executive officer (CEO), effective January 19, 2020. Barrett previously served as CardioFocus’ president and chief operating officer (COO).
The company is...
FEops has announced that the first patient has been enrolled in the physician-initiated PREDICT-LAA trial.
The trial is led by Righshospitalet (Copenhagen, Denmark) and aims to assess whether the use of FEops HEARTguide computer simulations based on cardiac CTimaging can...
Highlights:
WRAP-IT shows significant mortality risk with CIED infection, and reduced quality of life
Sequential approach using STAR mapping system identifies key early sites of activation in patients with persistent AF
Profile: Eric N Prystowsky
Andrew Grace discusses how...
Leading electrophysiologists have pinpointed four clinical studies due to report findings in 2020—AMULET IDE, aMAZE IDE, PULSED AF and CEASE AF—that they expect to shape practice in the treatment of cardiac arrhythmias this year.
Dhanunjaya Lakkireddy (chair of the American...
Andrew Grace charts the evolution of non-contact mapping culminating in the current AcQMap system—the technology has advanced understanding of persistent atrial fibrillation (AF), providing further support for the idea that AF is, in fact, a disorder of cardiac development.
Electromagnetic...