Highlights:
-Wearable defibrillator aids decision making on ICD need
-First-in-man study shows promise with leadless pacing
-Remote monitoring of ICD patients: What is the patients' opinion by Werner Jung
-Profile: Andreas Goette
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/21-Cardiac-Rhythm-News.pdf
Highlights:
- Renal denervation now under consideration for arrhythmias
- Watchman device is safe but efficacy debated
- Leadless technology: The next frontier of cardiac pacing
- Profile: John D Day
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/20-Cardiac-Rhythm_low-res.pdf
Etienne Aliot (Nancy, France) has always been fascinated by the work of surgeons. This fascination set him on his path through medical school. He has since been at the forefront of many electrophysiological research initiatives, including influential consensus documents...
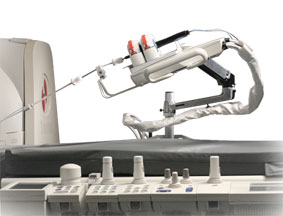
Stereotaxis is to present Niobe ES magnetic navigation system and supporting clinical evidence at Cardiostim-EHRA Europace 2016, taking place June 8-11 in Nice, France.
William C Mills, Stereotaxis chief executive officer, says, “This year, we have enhanced the ‘Stereotaxis experience’...
St Jude Medical’s CardioMEMS heart failure (HF) system has been added to the European Society of Cardiology (ESC) guidelines as a directed therapy management and monitoring tool for heart failure patients. The new 2016 ESC Clinical Practice Guidelines for...
The European Heart Rhythm Association (EHRA) is launching the patient- and caregiver-oriented website afibmatters.org in Swedish and Portuguese.
Afibmatters.org aims to contribute to improvements in the quality of life and health of patients with atrial fibrillation. The website is currently...
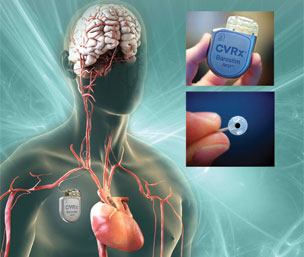
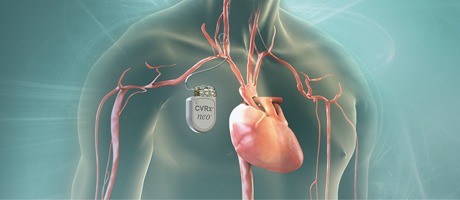
The first two patients have been treated with CVRx’s Barostim Neo system in the Baroreflex Activation Therapy for Heart Failure pivotal clinical trial (BEAT-HF). The procedures were done at the University of California San Francisco (San Francisco, USA) and...
The US Food and Drug Administration (FDA) has finalised its efforts to streamline the “compassionate use” process, used by physicians to access investigational drugs and biologics for patients with limited treatment options.
A statement from Robert Califf, the deputy...
Auris Surgical Robotics and Hansen Medical have signed a definitive merger agreement under which Auris will acquire Hansen Medical for US$4.00 per share in cash, or a total equity value of approximately US$80 million.
The transaction is expected to close...
A new bill introduced to the US Senate by Senator Dan Coats seeks to address the gap between the US Food and Drug Administration (FDA)’s priority review process for breakthrough medical devices, and the Centers for Medical and Medicare...
Occlutech has obtained European CE mark approval for its left atrial appendage, (LAA), occluder.
The device is a specifically designed implant for the minimally invasive closure of the LAA, a procedure that minimises the risk of strokes in patients...
Stereotaxis has announced the results of a retrospective study conducted at Paracelsus Medical University in Nuremberg, Germany, which showed significantly reduced radiation exposure for patients undergoing catheter ablation for persistent atrial fibrillation with the Niobe magnetic navigation system. The...
Pre-clinical data, presented at the 13th International Dead Sea Symposium on Innovations in Cardiac Arrhythmias and Device Therapy (IDSS; 6–9 March, Tel Aviv, Israel), have shown that it is feasible and safe to isolate pulmonary veins for the treatment...
There is a lack of a uniform approach or guidelines when dealing with the ethical, legal and privacy concerns that can arise in cardiogenetics. These concerns include “duty to warn” and “child protection” dilemmas and may occur in both...
New research indicates that catheter ablation may achieve greater long-term freedom from atrial fibrillation, as well as a reduction in unplanned hospitalisation and mortality in patients with heart failure and persistent atrial fibrillation.
In this open-label, randomised, parallel-group, multicentre clinical...
The Netherlands presidency of the European Council and representatives of the European Parliament have reached a political agreement on two draft regulations for medical devices. The new regulations are aimed at ensuring that medical devices and in vitro diagnostic...
The heart rate may be an indicator of a person’s life expectancy. A research team at the Technical University of Munich (TUM) (Munich, Germany) has to this end analysed an effect which at first seems paradoxical: Minor irregularities in...
Maria Lucia Narducci (Department of Cardiovascular Sciences, Catholic University of Sacred Heart, Rome, Italy) and others report in Europace that the presence of residual fibrous tissue—or “ghosts”—after transvenous lead extraction is associated with a significantly increased risk of death....
Syed Rafay A Sabzwari and Dhanunjaya Lakkireddy (Kansas City, USA) review the importance of obstructive sleep apnoea (OSA) in electrophysiology and cardiology at large, as a secondary impact to an underserved area of healthcare, which also provides practical implications...
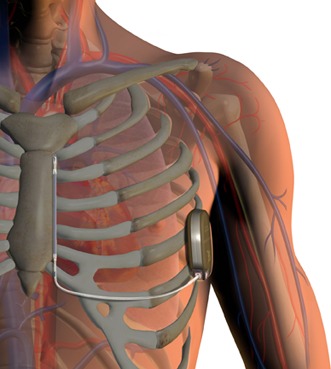
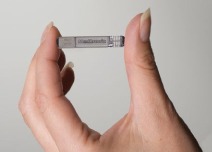
Biotronik has launched CardioMessenger Smart in the USA. CardioMessenger Smart is a portable monitoring device, about the size of a smartphone, which makes data from pacemaker, implantable cardioverter defibrillator (ICD), and insertable cardiac monitor (ICM) patients available remotely to...
A prospective, multicentre study—THERMOCOOL VT—analysing a cohort of non-randomised patients, published in February in the Journal of the American College of Cardiology, has shown that radiofrequency catheter ablation dramatically reduces ventricular tachycardia episodes and improves quality of life at...
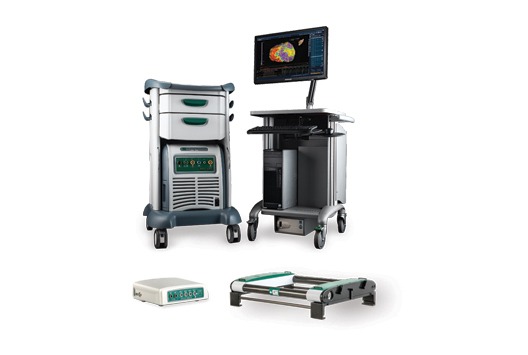
Catheter Robotics has completed the acquisition of a new product line to be named VIVO (View Into Ventricular Onset). VIVO is a computerised three-dimensional cardiac mapping system designed to show electrical activation of the ventricles of the heart.Catheter Robotics...
ResMed has announced primary results from a multicentre, randomised controlled phase II trial—CAT-HF—presented at the European Society of Cardiology’s 2016 Annual Heart Failure Congress.
CAT-HF assessed whether the treatment of moderate to severe sleep-disordered breathing (obstructive or central sleep apnoea)...
A randomised controlled study has found that yoga with light movements and deep breathing may lead to improved quality of life, as well as reduced blood pressure and heart rate in patients with paroxysmal atrial fibrillation. The study findings...
A subsequent study of the Value PVI (pulmonary vein isolation) study report several economic benefits, including reduced staff overtime and more time remaining for additional usage of electrophysiology laboratory resources with the use of cryoballoon ablation for atrial fibrillation...
New data from the EWOLUTION registry, presented at EuroPCR 2016, confirms safety of the Boston Scientific left atrial appendage closure system (Watchman). The data are for more than 1,000 patients, from across Europe, who received the device and focus on...
St Jude Medical has announced results from two cardiovascular clinical trials presented at EuroPCR 2016. The studies, which look at how St Jude Medical’s fractional flow reserve (FFR) technology impacts patient outcomes in acute coronary syndrome and a comparison...
Karl-Heinz Kuck, Asklepios Klinik St Georg, Hamburg, Germany, speaks at ACC 2016 about the FIRE AND ICE trial results which have shown that cryoballoon ablation is non-inferior to radiofrequency ablation in treating patients with drug-refractory paroxysmal atrial fibrillation, with...
Guilherme Fenelon (Sao Paulo, Brazil) overviews the role of ablation of ventricular tachycardia in Chagas disease. He says: catheter ablation in Chagas disease remains a “challenging procedure,” however, substrate mapping and ablation of ventricular tachycardia “is very useful for...
Corvia Medical has been granted CE mark approval for its InterAtrial shunt device (IASD). The IASD is a transcatheter device designed to treat heart failure with preserved ejection fraction (HFpEF), previously called diastolic heart failure.
David Muller, director of Cardiac...
Implantable cardioverter-defibrillators (ICDs) do not improve overall long-term survival compared to medical treatment in patients with non-ischaemic systolic heart failure, according to results of the DANISH clinical trial. These data prompted researchers and other physicians at the European Society...
OASIS, the first randomised, controlled, multicentre clinical trial to compare rotor-only ablation with two other ablation strategies in non-paroxysmal atrial fibrillation patients found “poor outcomes in terms of arrhythmia recurrence” with rotor-only ablation, for which enrolment on this treatment...
Studies presented at the 37th Heart Rhythm Society Scientific Sessions (HRS; 4–7 May, San Francisco, USA) have shown the feasibility of a novel approach to implantable cardioverter defibrillator (ICD) using Medtronic’s EV-ICD system. The studies were the first to...
Medtronic has announced clinical results highlighting the safety and performance profile of the miniaturised Micra transcatheter pacing system (TPS) at the 2016 Heart Rhythm Society meeting.
Several studies evaluating a novel approach to implantable cardioverter defibrillator (ICD) have shown the feasibility of therapy using Medtronic's EV-ICD system, according to a company release.
The research was presented the Heart Rhythm Society's 37th Annual Scientific Sessions, and includes data from both high volume and low volume medical centres.
Cell Therapy has granted the Japan license for its innovative cardiac regeneration medicine, Heartcel (immuno-modulatory progenitor (iMP) cells) to Daiichi Sankyo.
Acutus Medical has received CE mark approval for its AcQMap high resolution imaging and mapping system, and for its AcQMap catheter.
Data collected from the EFFORTLESS study were presented as a late-breaking clinical trial at the 37th Annual Scientific Sessions of the Heart Rhythm Society (HRS) in San Francisco, USA.
A multicentre, randomised controlled trial has shown that catheter ablation is superior to intensified antiarrhythmic drug therapy in reducing death, appropriate implantable cardioverter defibrillator (ICD) shock and ventricular tachycardia storm in patients with ischaemic cardiomyopathy, with an ICD who...
Results from the AFACT trial have shown no clinical benefits and significantly more complications associated with routine ganglionic plexus ablation for advanced atrial fibrillation patients. Data were presented at the 37th Heart Rhythm Society Scientific Sessions (HRS; 4-7 May, San Francisco, USA).
Results of St Jude Medical's MultiPoint Pacing investigational device exemption clinical study have been presented during a late-breaking clinical trial session at the Heart Rhythm Society's (HRS) 37th annual scientific sessions.
The European Heart Rhythm Association
(EHRA) invites EHRA current and past winners of the Training Fellowship Programmes or established physicians wanting to acquire additional techniques/skills in the field of arrhythmias or cardiac pacing to apply for the EHRA Proctor...
Boston Scientific has received US Food and Drug Administration (FDA) approval for two catheters that can be used with the company's Rhythmia mapping system.
Stereotaxis will share results of recently published clinical studies, new technology enhancements and simulations of its computer-controlled mapping and lesion formation capabilities at HRS 2016.
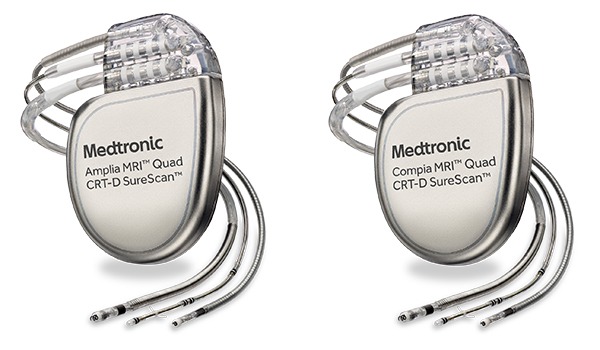
The Iperia ProMRI HF-T cardiac resynchronisation defibrillator has been approved by the US Food and Drug Administration (FDA).
Merit Medical Systems has officially launch its Interventional cardiac resynchronisation therapy initiative during the Heart Rhythm Society (HRS) Meeting in San Francisco, USA.
George Van Hare (The Louis Larrick Ward professor of Pediatrics and director, Division of Pediatric Cardiology, Washington University School of Medicine, St Louis, USA), has worked towards advancing paediatric electrophysiology, as a recognised specialty, in the paediatric cardiology world and the electrophysiology world. He considers that working as a paediatric electrophysiologist is a "rewarding" experience, because it has allowed him to diagnose and cure at a very early stage. He talks to C
Abbott is set to acquire St Jude Medical, expanding its portfolio to cover cardiovascular markets such as atrial fibrillation, structural heart and heart failure as well as neuromodulation. The combined company will thus produce devices across cardiovascular, diabetes, vision...
Medtronic has announced it has received US Food and Drug Administration (FDA) approval for the Visia AF MRI SureScan and Visia AF single-chamber implantable cardioverter defibrillators (ICDs).
Boston Scientific has announced key data, including one late-breaking clinical trial, that will be featured at the 37th Annual Scientific Sessions of the Heart Rhythm Society (HRS) in San Francisco on May 4-7 2016.
Medtronic has announced one-year results from a real-world study of patients who had a cryptogenic stroke, or stroke of unknown cause.
Boehringer Ingelheim and Eli Lilly are to conduct two outcome trials investigating the diabetes medicine empagliflozin (Jardiance) for the treatment of people with chronic heart failure.
Boston Scientific has received CE mark approval for the new Emblem MRI subcutaneous implantable defibrillator (S-ICD) system, as well as magnetic resonance (MR) conditional labelling for all previously implanted Emblem S-ICD systems.
The 75th patient has been enrolled in GENETIC-AF, a phase 2B/3 clinical trial evaluating bucindolol (Gencaro, Arca Biopharma) as a potential treatment for atrial fibrillation.
St Jude Medical has announced expansion of its EnSite Precision cardiac mapping system limited market release in Europe and use of the new platform in more than 600 cases in nine countries since receiving CE mark in January 2016.
AtriCure has received US Food and Drug Administration 510(k) clearance for the cryoFORM cryoablation probe, which is designed to offer increased probe flexibility to adapt to a variety of surgical cardiac ablation procedures.
Healthcare professionals performing x-ray guided cardiovascular procedures may be at higher risk for health problems including orthopaedic problems, cataracts, skin lesions and cancers, according to new research.
Biotronik has announced Food and Drug Administration (FDA) approval of BioMonitor 2, an insertable cardiac remote monitor with ProMRI technology.
Results from the INOVATE-HF trial indicate that vagus nerve stimulation does not reduce the rate of death or heart failure events in chronic heart failure patients. The data were simultaneously presented at ACC 2016 and published in the Journal of the American College of Cardiology.
According to a Reuters news report, Boston Scientific is temporarily suspending sales of Watchman FLX—the next-generation of its left atrial appendage closure device, Watchman. The news agency says the company is suspending sales in Europe because of a higher-than-expected...
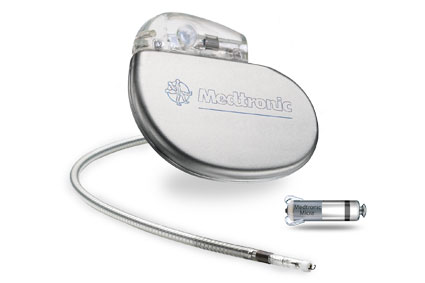
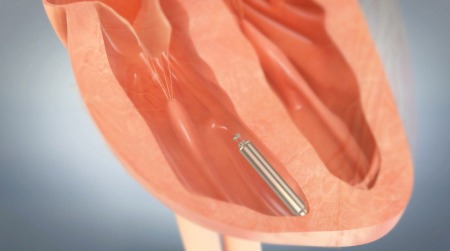
Medtronic has announced it has received US Food and Drug Administration (FDA) approval for its Micra Transcatheter Pacing System (TPS). The leadless device, which is 93% smaller than conventional pacemakers, provides a safe alternative for pacing without the complications associated with cardiac leads.
Richard Chazal has assumed his role as president of the American College of Cardiology (ACC) during the Convocation Ceremony held in conjunction with the ACC's 65th Annual Scientific Session in Chicago.
A Allen Seals has been elected chair of the American College of Cardiology (ACC) Board of Governors and secretary of the Board of Trustees, the main governing body of the ACC, for 2016-2017.
Portola Pharmaceuticals has announced that it has entered into a clinical collaboration agreement with Daiichi Sankyo to develop andexanet alfa as an antidote for edoxaban, Daiichi Sankyo's Factor Xa inhibitor, in Japan.
Many patients who have an implantable cardioverter defibrillator (ICD) are unaware that the device can be deactivated to prevent painful shocks in their final days of life, according to two new studies.
Northwell Health physicians presented data at the American College of Cardiology 65th Annual Scientific Session (2-4 April, Chicago, USA) showing heart failure management with the CardioMEMS HF System leads to significant improvements in quality of life and exercise capacity for patients with heart failure.
CardioFocus has announced that it has received premarket approval from the US Food and Drug Administration (FDA) for its HeartLight Endoscopic Ablation System for the treatment of patients with paroxysmal atrial fibrillation.
Stereotaxis has announced that Takatsuki General Hospital in Japan has reached the milestone of treating 120 patients using the company's Niobe remote magnetic navigation system. This milestone completes the required patient enrolment for the Niobe system's post-market surveillance in Japan.
Researchers in Austria have found shorter time to sinus rhythm in patients with recent-onset atrial fibrillation (AF) treated with intravenous vernakalant (Brinavess, Cardiome Pharma) compared with ibutilide treatment.
The 4WARD Coalition has launched AFib4WARD.com, an online tool designed to help non-valvular atrial fibrillation patients and their healthcare providers engage in informed discussions and shared decision making.
A new test to identify patients at risk of sudden cardiac death is being developed by researchers at the University of Leicester and Leicester's Hopsitals following a £183,000 grant from national charity Heart Research UK.
The first patient has been enrolled in the SynCor clinical trial of Xenios’ CE-marked i-cor synchronised cardiac assist system for treating cardiogenic shock.
The SynCor trial is a prospective, non-randomised, multi-centre, open-label observational study of the safety and performance of...
St Jude Medical has announced the launch and CE mark approval for three new Quartet left ventricular (LV) leads.
New research published in the Journal of the American College of Cardiology indicates that a wide/and or large S-wave in lead I is a powerful predictor of life-threatening ventricular arrhythmias in Brugada Syndrome patients with no history of cardiac arrest at presentation.
A wearable automatic defibrillator may be an option for patients who are at risk for life-threatening heart rhythm abnormalities but are not good candidates for an implantable cardiac defibrillator, according to an advisory for the American Heart Association (AHA).
Zoll Medical has announced that its Japanese subsidiary, Asahi Kasei Zoll Medical, has obtained approval from Japan's Pharmaceuticals and Medical Devices Agency (PMDA) to market the company's Thermogard XP.
The European Heart Rhythm Association (EHRA) invites its current members to renew their memberships for 2016 and invites cardiac rhythm management and electrophysiology experts, physicians and allied professionals who are not members yet to join. The Association also announces...
The US Food and Drug Administration has announced a proposal to ban most powdered gloves in the USA. While use of these gloves is decreasing, they pose an unreasonable and substantial risk of illness or injury, according to an FDA news release.
Sir Nilesh Samani has been announced as the next medical director of the British Heart Foundation. He will succeed Peter Weissberg who will retire in October 2016.
Nearly half of all atrial fibrillation patients at the highest risk for stroke are not being prescribed blood thinners by their cardiologists, according to a new study.
Lindsay Anderson (Duke Clinical Research Institute, Duke University Medical Center, Durham, USA) and others report in the American Heart Journal that, compared with medical management, percutaneous coronary intervention (PCI) is not associated with a significant reduction hospital readmission for...
AliveCor has introduced the first medical-grade Electrocardiogram (EKG) band for the Apple Watch, the Kardia Band, along with a new app for smartphones.
The UK National Institute for Health and Care Excellence (NICE) has recommended sacubitril/valsartan (Entresto) in its final draft guidance for use within the UK National Health Service.
Arca Biopharma has announced the GENETIC-AF trial, which will evaluate bucindolol (Gencaro) as a potential treatment for atrial fibrillation.
InfoBionic has received 510(k) clearance from the US Food and Drug Administration (FDA) for MoMe Kardia, a wireless, remote monitoring system.
A new bidirectional rotational mechanical lead extraction sheath is both safe and effective in performing lead extraction, according to a study published online ahead-of-print in Europace. Clinical success for the leads extracted using this novel sheath was 98.1% with no mortality or major complications.
A real-world study has found that the SmartTouch (Biosense Webster) contact force-sensing catheter coupled with an Advanced Catheter Location feature during atrial fibrillation (AF) ablation reduced fluoroscopy times by 77%, radiation dose by 71% and procedural time by 19%.
BioMonitor 2 (Biotronik) is now available for patients in the UK and Ireland. The insertable device is designed to allow accurate and reliable continuous detection of cardiac electrical events.
The approval of the Blazer OI catheter marks the first time Boston Scientific will offer an open-irrigated catheter to the US market.
CE mark approval has been secured for the magnetic resonance (MR) conditional labelling for 1.5T scans for the Nanostim leadless pacemaker from St Jude Medical.
Zoll Medical has announced a new chief executive officer and senior vice president of resuscitation as Richard A Packer is to lead Asahi Kasei's Healthcare Business Unit.
The first commercial implantations of WiSE (wireless stimulation endocardially) technology (EBR Systems) have taken place in the UK and Czech Republic.
Patients with hypertrophic cardiomyopathy at risk of sudden cardiac death and without pacing indication may be eligible for Subcutaneous Implantable Cardioverter Defibrillator (S-ICD) implantation, according to results of a single-centre study.
Shlomo Ben-Haim (London, UK), a professor of medicine and serial entrepreneur in the medical device industry, examines the drivers of expansion of the atrial fibrillation (AF) ablation market, the existent barriers to market penetration and explains how efficiency and...
The system produces real-time 3D catheter location information from 2D fluoroscopic images of the heart, and correlates them with the electrical activation of the heart.
The US Food and Drug Administration (FDA) has confirmed the appointment of Robert Califf as its 22nd commissioner, following a US Senate vote of 88 to 4 in his favour.
Califf was nominated to replace previous commissioner, Margeret A Hamburg, by...
Biotronik has opened the Education and Innovation Center in New York, USA to hold educational programmes.
The US Food and Drug Administration has granted approval to Boston Scientific for its Acuity X4 quadripolar left ventricular leads. The company can now offer its first full X4 cardiac resynchronisation system to the US market.
The three cardiac resynchronisation therapy defibrillators (CRT-Ds) are approved for 3 Tesla magnetic resonance imaging scans. These devices are the first and only CRT-Ds approved for this level of MRI, according to a company release.
The FDA's Circulatory System Devices Panel of the Medical Devices Advisory has made recommendations for leadless pacemakers regarding adverse events, long-term safety issues (including battery longevity), necessary elements for postmarket surveillance, indications for use and labelling, and implanting physicians' training.
The panel provided insight around patient selection and post approval study methodology, folowing a panel discussion on leadless pacing technology.
Studies have shown that heart failure affects African American individuals with roughly twice the incidence of that of Caucasians. The Hispanic population has the second-highest risk of developing heart failure in the USA.
The American College of Cardiology has release its 2016 ACC Lifelong Learning Competencies for General Cardiologists. This document defines the knowledge, skills and behaviours expected of practising clinical cardiologists.
The agreement of the English National Institute for Health and Care Excellence, the All Wales Medicines Strategy Group and the Irish National Centre for Pharmoeconomics has granted the drug eligibility for full reimbursement, without the need for a full appraisal in these countries.
The first patient has been enrolled in the ATLAS (AtriClip Left Atrial Appendage Exclusion Concomitant to Structural Heart Procedures) clinical study.
This technology is designed to provide additional options which may benefit cardiac resynchronisation therapy patients who are not responsive to other methods of pacing.
Researchers in Turkey have found that additional left atrial appendage (LAA) isolation using second generation cryoballoon technology is feasible and safe and that it may be considered as an adjunctive therapy to pulmonary vein isolation for persistent atrial fibrillation (AF) treatment.
Despite major advances in techniques and technology over the past 15 years, the clinical outcomes for catheter ablation in non-paroxysmal atrial fibrillation patients remain disappointing, write Tom Wong and Shouvik Haldar (London, UK). The authors review data which favour...
Left ventricular (LV) lead implantation for cardiac resynchronisation therapy (CRT) with a robotically-guided surgical approach through the coronary sinus seems to offer a new alternative when conventional approaches are not suitable, a new study has found.
The company will concentrate on developing new technologies to aid in the advancement of cardiovascular medicines using stem cell therapy.
The Japanese launch of the device took place in July 2015. This is the first and only CRT-D in Japan with such conditions, according to a company release. All already implanted devices have been deemed safe for full-body MRI scans at 1.5 tesla strength.
The winners of the European Society of Cardiology competitive research programme ‘Grants for Medical Research Innovation’ have been announced. The grants are awarded to research projects that will address areas of unmet medical need in thromboembolic disease.
“We were astounded...
Members of the FDA's Circulatory System Devices Panel of the Medical Devices Advisory Committee will meet on 18 February 2016 to provide advice and recommendations on leadless cardiac pacemaker device technology.
The US Centers for Medicare and Medicaid Services (CMS) are to cover percutaneous left atrial appendage closure (LAAC) therapy under specific criteria, as outlined in the agency’s final National Coverage Determination (NCD). This decision, effective immediately, provides consistent and...
The company has received premarket notification 510(k) clearance for the Bridge Occlusion Balloon for lead extraction procedures. This clearance will initiate a controlled market release, according to a Spectranetics release, with full market launch at the Heart Rhythm Society's 37th Annual Scientific Sessions.
Medtronic has announced that it is the first company to receive US Food and Drug Administration approval for magnetic resonance imaging (MRI) conditional cardiac resynchronisation therapy (CRT) defibrillators for the treatment of heart failure.
MYK-461, a drug candidate from MyoKardia, may prevent and reverse development of hypertrophic cardiomyopathy in multiple genetic mouse models, according to a study published in Science.
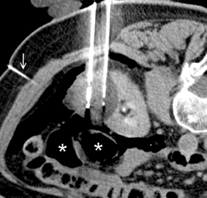
An animal study from China has indicated that it is feasible to implant two leadless pacemakers in the right ventricle of the same heart without impacting cardiac function at six months.
The battery life of cardiac implantable electronic devices must be improved to reduce the need for replacement and the risks this carries for patients, argue UK cardiologists John Dean and Neil Sulke in an editorial published in BMJ.
Kalila Medical has a novel steerable sheath that is designed to help physicians more easily access and perform catheter-based electrophysiology procedures.
Portola has also entered into a clinical collaboration agreement with Bayer HealthCare to include its Factor Xa inhibitor rivaroxaban in this clinical development programme in Japan.
Ilivia devices come with the company's ProMRI technology, as well as MRI AutoDetect, which is designed to allow the cardiologist to activate a window in which all device functionality is maintained until a patient actually undergoes an MRI scan.
Boston Scientific and Accenture are to launch a cloud-based, data-driven healthcare system for hospitals. The system, called Advantics Care Pathway Transformation, is designed to help improve patient outcomes and reduce costs to treat patients with chronic cardiovascular diseases.
The platform...
This trial evaluates bucindolol hydrochloride (Gencaro) as a potential genetically-targeted treatment for the prevention of atrial fibrillation.
A previously communicated voluntary global field safety action related to St Jude Medical's Optisure Dual Coil Defibrillation Leads has now been classified as a Class 1 Advisory by the US Food and Drug Administration.
Stereotaxis and Philips have signed an addendum, pursuant to their existing development and cooperation agreement, to facilitate development of a new interface between each company's most advanced systems for electrophysiology and interventional cardiology procedures.
The German Federal Joint Committee (Gemeinsamer Bundesausschuss-G-BA) has granted Daiichi Sankyo's edoxaban (Lixiana) an indication of a minor additional benefit.
AtriCure has received approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for its AtriClip products, which will be distributed in Japan by Century Medical.
Sacubitril/valsartan (Entresto, Novartis) has been made available in the UK for the treatment of adult patients with symptomatic chronic heart failure with reduced ejection fraction.
Chu-Pak Lau explores the development and use of externally-powered and entirely intracardiac leadless pacemakers, and considers the effect they have had on clinical practice.
A leading heart rhythm expert in Australia, Jonathan M Kalman directs both clinical and research groups in the Department of Cardiac Arrhythmias at the Royal Melbourne Hospital and University of Melbourne, Australia. In this interview, he recalls details of the first curative ablation procedure for atrial fibrillation in Australia and talks about the impact of lifestyle modification in atrial fibrillation management.
An analysis from the US Get with the guidelines for heart failure registry has found that women and men with heart failure and reduced left ventricular ejection fraction benefit similarly from implantable cardioverter defibrillators.
The Advantage-MR EP recorder/stimulator system is a magnetic resonance conditional recording system for magnetic resonance imaging-guided electrophysiology procedures.
The recently-obtained data demonstrates continuing and steady progress for the drug candidates in Verseon's anticoagulation program, according to a press release.
AliveCor are to partner with LifeWatch AG. AliveCor Mobile ECG will be integrated into LifeWatch's remote cardiac monitoring service. This represents AliveCor's first step into remote patient monitoring.
Stereotaxis has initiated its first prospective, multicentre, randomised clinical study to compare radiofrequency ablation outcomes generated using its Niobe ES remote magnetic navigation system to manual approaches in ischaemic scar ventricular tachycardia (VT) patients.
Mark W Kroll (Minneapolis, USA) writes about the importance of optimisation of implantable cardioverter defibrillators (ICDs) in the absence of defibrillation threshold testing.
Initial findings have shown that a new ultrasound-based imaging system with continuous dipole density mapping provides real-time rapid global left atrial reconstruction and compares favourably to segmented computed tomography (CT).
The Advanced Medical Technology Association (AdvaMed), the Medical Imaging & Technology Alliance (MITA) and the Medical Device Manufacturers Association (MDMA) have applauded the US Congress for their passage of a two year suspension of the medical device excise tax,...
Biotronik has been granted US Food and Drug Administration approval for use of a group of implantable cardioverter defibrillator systems with magnetic resonance imaging scans.
Scoring highly on the American Heart Association (AHA)'s Life's Simple 7 checklist has been associated with a reduction in heart failure risk, according to a study published Circulation: Heart Failure.
LifeWatch Services has acquired FlexLife Health, a company which offers patients remote services to monitor and measure coagulation.
Portola Pharmaceuticals have completed the submission of a Biologics License Application for its investigational agent andexanet alfa to the US Food and Drug Administration.
The LuxCath optical tissue interrogation technology was used in eleven patients suffering from arrhythmias such as atrial flutter, AV nodal re-entrant tachycardia, and atrial fibrillation.
LiveVest is now approved for use by certain children who are at risk for sudden cardiac arrest, but who are not candidates for an implantable defibrillator device
The first patient has been enrolled in the CEASE (combined endoscopic epicardial and percutaneous) atrial fibrillation (AF) clinical study, according to a press release from AtriCure.
Dhiraj Gupta (Liverpool, UK) overviews developments in two left atrial appendage (LAA) occlusion devices, which have helped make the implant procedure safer, easier and quicker. He also highlights current challenges related to overall peri-procedural patient management.
This update-which has been produced since 1958-is made up from the most-recent data available compiled by the AHA, the National Institutes of Health, the Centers for Disease Control and Prevention and other government sources.
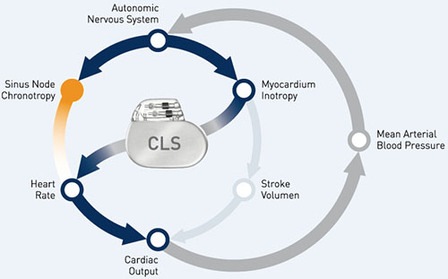
BIOSync CLS will investigate the efficacy of the uniquely physiologic rate response sensor CLS in preventing syncope.
The device from Spectranetics is designed to substantially reduce blood loss in the event of a superior vena cava tear.
The device enables the clinician to access real-time streaming for live patient visibility, auto-detection of arrhythmia events, and wireless transmission of three channels of ECG data.
HeartRescue is a Medtronic Philanthropy partnership launched in five US states in 2010, which aims to improve Sudden Cardiac Arrest (SCA) survival rates.
Cardiac resynchronisation therapy with defibrillator may prevent hospitalisation due to heart failure, when compared to treatment with implantable cardioverter defibrillator alone.
Biotronik is to support its charity partner, Heartbeat International (HBI) at the 25th Interamerican Congress of Cardiology, in Santiago, Chile.
A strategy including insertable cardiac monitors (ICM) to guide rhythm control with antiarrhythmic drugs and assessment of AF burden may allow safe discontinuation of oral anticoagulation in AF patients at high risk of bleeding.
The device is approved for use during 1.5 Tesla full-body MRI scans and ultra-high strength 3.0 Tesla MRI scans with an exclusion zone.
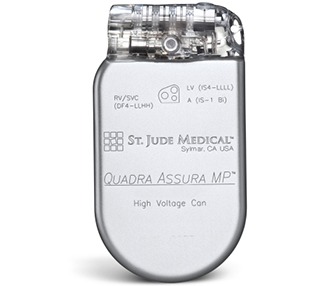
The Quadra Assura cardiac resynchronisation therapy defibrillator (CRT-D) is now approved for use with magnetic resonance imaging (MRI) scanning systems at 1.5 Tesla.
Leading heart failure doctors from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) have united in a pledge to improve patient outcomes and reduce the burden of heart failure on society.
Medtronic has reported an issue with the long-term battery performance of its InSync III cardiac resynchronisation therapy-pacemakers (CRT-P) (models 8042, 8042B and 8042U). The Food and Drug Administration (FDA) has designated this issue as a Class II recall.
This trial aims to determine if closed loop stimulation can delay the onset of atrial fibrillation and reduce the risk of stroke.
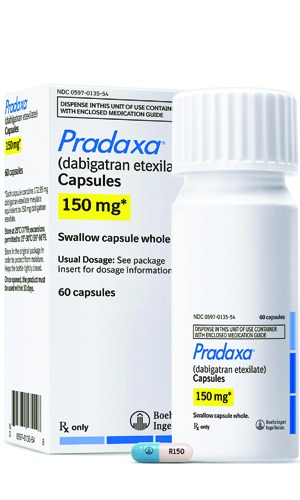
Boehringer Ingelheim has announced that the European Commission has licensed idarucizumab (Praxbind) for rapid and specific reversal of the anticoagulant effects of dabigatran etexilate in cases of emergency surgery/urgent procedures or in situations of life-threatening or uncontrolled bleeding.
The results were published online by The New England Journal of Medicine, while the ANNEXA-R data were presented during a late-breaking clinical trial session at AHA Scientific Sessions 2015.
Novartis' Entresto (sacubitril/valsartan) has been authorised for the treatment of adult patients with symptomatic chronic heart failure with reduced ejection fraction (HFrEF).
These global recommendations are from The Heart Rhythm Society (HRS), European Heart Rhythm Association (EHRA), Asia Pacific Heart Rhythm Society (APHRS), and the Socieded Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLAECE).
A study has shown heart failure, diabetes and recent heart attacks to be the strongest predictors of stroke caused by a blocked artery.
This acquisition adds the Coherex WaveCrest left atrial appendage (LAA) occlusion system to BioSense's portfolio.
This award recognises Ebit's Suitestensa cardiovascular information system (CVIS) integration platform.
The Micra pacemaker was chosen from thousands of submissions, and is one of among 100 honourees that are seen represent a significant leap forward in their respective categories by the magazine.
According to Medtronic, this is the world's first app-based remote monitoring system for patients with implantable pacemakers.
The results of an independent, multicentre study, which looked at the procedural benefits and outcomes of patients undergoing radio frequency ablation therapy for ventricular tachycardia, were published at the AHA.
Josep Brugada (Barcelona, Spain), Carlo Pappone (Milan, Italy) and others report in Circulation: Arrhythmia and Electrophysiology that ablation of abnormal epicardial substrate in patients with Brugada syndrome can eliminate the phenotype expression of the syndrome.
The new device is a subcutaneous, insertable cardiac remote monitor designed to continuously monitor
cardiac electrical events reliably and accurately.
By Martin W Bergmann
Martin W Bergmann is an interventional cardiologist working in Germany, who performs percutaneous left atrial appendage (LAA) closure procedures. He explores why there is still a need for such procedures even in the era of novel...
Vic Gundotra has worked as a senior vice president at Google, after spending 15 years with Microsoft.
The symposium is an educational program for electrophysiologists and device-oriented cardiologists.
Concerns for both patients and their partners declined after three months.
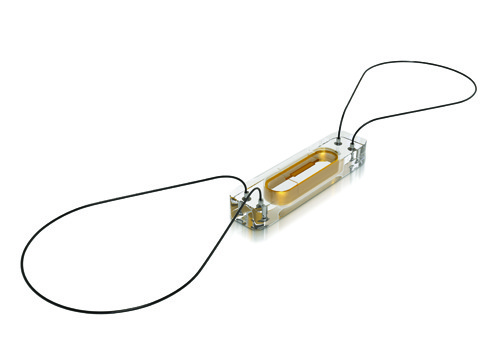
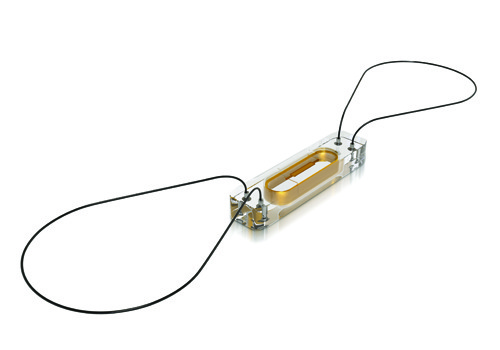
The transcatheter pacing system from Medtronic is the smallest pacemaker on the market.
The meta-analysis-which covered 33 studies involving more than 20 million patients-has been published in the Journal of the American College of Cardiology.
Despite the lack of randomised trials on energy drinks consumption, few case reports on atrial and ventricular arrhythmias as well as on myocardial ischaemia inducted by energy drinks have raised concerns on the safety of these beverages; write Alessandro...
Speaking at a debate about the value of defibrillator threshold testing in patients with ICDs, Klaus Witte (Leeds, UK) told delegates at the Heart Rhythm Congress that such testing was a "pointless" intervention because it does not provide benefit and may be associated with adverse events. Nick Linker (Middlesbrough, UK), who argued against Witte, said that there is still a significant proportion of patients-who were not included in the major trials of defibrillation testing-for whom
Platinum is designed to protect patients from avoidable replacement surgeries and the inherent risk of complications.
The recommendations from the American Heart Association and the American College of Cardiology may permit participation in competitive sports for some athletes with long QT syndrome
The European Heart Rhythm Society (EHRA) and Heart Failure Association (HFA) have initiated the European Cardiac Resynchronization Therapy Survey II, an initiative designed to collect information on the delivery of cardiac resynchronisation therapy (CRT), including indications, in a large...
The implantation of an implantable cardioverter defibrillator (ICD) device poses numerous psychosocial challenges, which have been shown to significantly influence functional outcomes in this population. Therefore, the optimisation of medical therapy and provision of psychological support is key to managing the patient's biopsychosocial functioning, write Elizabeth Banwell, Katie Murray and Stephen Gunning.
Blackouts and near drownings may point to long QT syndrome (LQTS), signalling an increased risk of sudden death, according to research presented at the SA Heart Congress (25-28 October, Sun City, South Africa) by Paul Brink, Tygerberg, South Africa.
The Visia AF and Visia AF MRI SureScan are designed to detect and monitor new onset, asymptomatic and previously undiagnosed atrial fibrillation.
According to research presented at Acute Cardiovascular Care 2015, pacemaker checks are a good way to identify new cases of atrial fibrillation so that anticoagulation can be started to prevent strokes.
Idarucizumab (Praxbind) is the first reversal agent approved specifically for dabigatran and works by binding to the drug compound to neutralise its effect.
The American Heart Association (AHA) and Heart Rhythm Society (HRS) have announced a collaboration designed to improve the quality of care of atrial fibrillation (AFib) patients and advance to cardiovascular research.
St Jude Medical has announced its completion of the previously announced acquisition of Thoratec Corporation, a global leader in mechanical circulatory support technology for the treatment of advanced heart failure.
Regulatory approval has been granted in South Korea for the Parachute System by CardioKinetix by the Korean Ministry of Food and Drug Safety (MFDS).
Michael Glikson (Tel Hashomer, Israel) has contributed to the development of technologies for CRT, modern lead extraction, advanced mapping ablation of AF and VT and LAA occlusion. Glikson's current projects as president of the Israel Heart Society and co-president of the International Dead Sea Symposium (IDSS) on Innovations in Cardiac Arrhythmias and Device Therapy reflect his innovative approach in the field. He speaks to Cardiac Rhythm News about these projects, other highlights in his
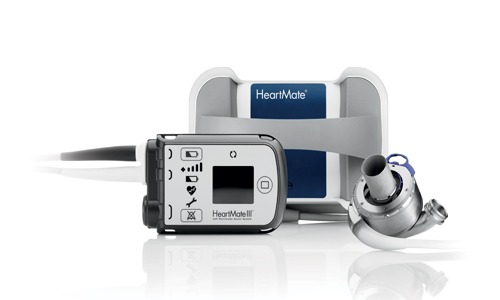
St Jude Medical has announced that it has received CE mark approval for the HeartMate 3 Left Ventricular Assist System (LVAS). This is a cardiac support option for advanced heart failure patients awaiting transplantation who are not candidates for heart transplantation, or in myocardial recovery.
In recently published studies, modulation of rotors by ablation seems to be effective in termination of atrial fibrillation. Now, researchers are exploring the role of rotors in ventricular fibrillation. Siva K Mulpuru (Mayo Clinic, Rochester, USA), discusses the latest research in this field.
AtriCure has entered into a definitive merger agreement under which it will acquire nContact, a privately-held developer of innovative cardiac ablation solutions.
EBR Systems has announced the CE mark approval for its Wise (Wireless stimulation endocardially) technology, which is the world's only wireless endocardial pacing system for cardiac resynchronisation therapy (CRT).
Atricure has announced the launch of the Cryoform cryoablation probe, which offers increased probe flexibility to adapt to a variety of surgical ablation procedures.
Renato P Ricci (Rome, Italy) writes about the HomeGuide organisational model, a strategy aimed to improve the effectiveness of remote monitoring of cardiac implantable electronic devices (CIEDs) with low resource consumption. The strategy focuses on the joint work of...
More real-world safety data could potentially address the issue of under-dosing and under-treatment with novel oral anticoagulants (NOACs) in global practice, says an analyst with research and consulting firm GlobalData.
Data show that autonomic regulation therapy in patients with moderate to severe chronic heart failure and impaired heart function is well tolerated, safe, improves the heart's ability to pump blood, and reduces the frequency and severity of symptoms associated with chronic heart failure.
John Dahldorf, Martin Chambers and Steven McQuillan join the company as chief financial officer, chief commercial officer and senior vice president, Regulatory and Clinical Affairs, respectively.
Pending final approval by the European Commission, Entresto (sacubitril valsartan) will be licensed for use in the UK for the treatment of adult patients with symptomatic chronic heart failure and reduced ejection fraction.
The European Medicines Agency (EMA) has recommended granting a marketing authorisation, following accelerated assessment, for idarucizumab (Praxbind) as a specific antidote to dabigatran etexilate (Pradaxa).
The National Institute for Health and Care Excellence (NICE), the medicines cost-effectiveness body for England and Wales, has recommended a new treatment to help prevent stroke and systemic embolism in patients suffering from atrial fibrillation.
A new guideline aimed at helping clinicians treat patients with supraventricular tachycardia has been released by the American College of Cardiology, American Heart Association, and Heart Rhythm Society.
Due to the complex nature of clinical cardiac electrophysiology, an updated training statement released by the American College of Cardiology, the American Heart Association and the Heart Rhythm Society, is calling for increased training for practitioners.
Financial details of the transaction are not being released. The combination creates one of the world's largest AED solutions providers.
The second part of Portola Pharmaceuticals' phase 3 ANNEXATM-R study achieved all primary and secondary endpoints with high statistical significance.
The study, known as RESTORE SR, will enrol 600 subjects at trial sites in the USA and other countries and will evaluate vanoxerine at a 400mg dose.
The accepted PMA application includes safety and effectiveness data from the company's multicentre HeartLight pivotal clinical trial, a randomised, controlled study in which a total of 353 patients were treated at 19 US centres.
Medtronic has announced that it has received the first US Food and Drug Administration (FDA) approval for an implantable cardioverter defibrillator (ICD) system for use with magnetic resonance imaging (MRI) scans.
This two-by-two factorial, randomised controlled trial will evaluate the safety of Eliquis versus warfarin or other vitamin K antagonists (VKA) in patients with NVAF and a recent acute coronary syndrome or undergoing PCI.
Researchers at Thomas Jefferson University, Philadelphia, USA, have showed that a simple questionnaire, evaluation and pulse-oximetry monitoring can lead to early detection of sleep apnoea in patients hospitalised for congestive heart failure.
A randomised, multicentre study has found improvement in the long-term freedom from long-standing persistent atrial fibrillation (AF) in patients who underwent an ablation plus empirical electrical left atrial appendage isolation strategy. No major complications were reported.
These two-year prospective outcomes from cohorts 1 and 2 show a mortality rate of 3.83% per person year compared to stroke rates of 1.25% per person year and major bleeding rates of 0.7% per person year.
The new guidelines now recommend that subcutaneous defibrillators (S-ICDs) should be considered as an alternative to transvenous defibrillators in patients with an indication for an ICD when pacing therapy is not needed.
The studies compared the risk of different bleeding related outcomes, including major bleeding and/or any bleeding, hospitalisation and bleeding-related 30-day readmissions in routine clinical practice setting for apixaban versus warfarin, rivaroxaban and dabigatran.
Results from the real-world study XANTUS have shown low rates of major bleeding in patients with atrial fibrillation taking rivaroxaban (Xarelto, Bayer Healthcare) for stroke prevention. Data were consistent with findings from the pivotal phase III clinical trial, ROCKET-AF.
LCZ696 (sacubitril valsartan) will be made available to eligible patients in the UK before a final European licensing decision is made.
The next generation of electrocardiogram (ESC) solutions can be run with a smartphone or tablet PC, four electrodes and the CardioSecur App enabling the diagnosis and localisation of cardiac ischaemia, rhythm disorders and posterior wall infarctions.
A first-of-its-kind study indicates that gestational and early life secondhand smoke exposure may double one's chance of developing atrial fibrillation as an adult.
First-of-its-kind findings from two independent studies have identified a gene associated with sudden cardiac death.
The study about gender differences in athletes' hearts highlights the importance of understanding how women's hearts work, and that what looks normal in men could reveal problems in women.
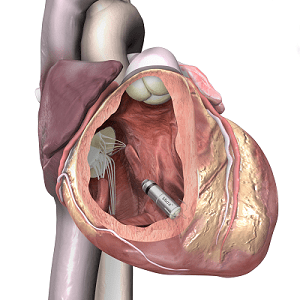
Primary results from the LEADLESS II study have shown positive efficacy and safety outcomes with the Nanostim leadless pacemaker (St Jude Medical) for patients who require a single-chamber ventricular pacemaker.
Study reveals how physical exertion may trigger sudden death in people with apparently healthy hearts.
The ESC Grants for Medical Research Innovation programme will see four grants of up to €400,000 each awarded to researchers or clinicians following live presentations by selected finalists to a panel of high profile experts appointed by the ESC.
INOVATE-HF has enrolled a total of 725 patients at 86 centres in the USA and Europe, making it the largest prospective, randomised device study to evaluate the treatment of heart failure with vagus nerve stimulation.
Stereotaxis and Westmead Hospital in Australia have announced findings of a study comparing the stability of a Niobe remote magnetic navigation system catheter group and a manually controlled catheter group in a validated cardiac wall motion simulator.
The largest study to date exploring the mid-term efficacy of cryoablation with the second generation device has shown favourable outcomes in patients with persistent atrial fibrillation. At a median follow-up of 10 months, 67% of patients were in sinus rhythm.
Biotronik has announced CE mark approval and the results of a pilot study into the performance of the subcutaneous, insertable cardiac monitor BioMonitor 2.
Biotronik has announced CE mark approval for its last two generations of ProMRI cardiac resynchronisation therapy defibrillator (CRT-Ds) systems. The approval allows full-body MRI scanning.
A European Society of Cardiology (ESC) position paper published in European Heart Journal outlines how the ESC will exploit e-health in education and research, while tackling issues of quality control and data security.
This revised labelling ensures that future patients and those already implanted with these systems are able to undergo MRI scans if indicated.
A satellite symposium and four GARFIELD-AF presentations will demonstrate how antithrombotic treatment patterns are evolving in the real-world and the impact on clinical outcomes in newly diagnosed atrial fibrillation patients.
The programme, which starts in September during Atrial Fibrillation Awareness Month, aims to reach women living with heart disease who do not otherwise have access to patient support services.
Smartphone applications and wearable sensors have the potential to help people make healthier lifestyle choices, but, according to the American Heart Association, scientific evidence of mobile health technologies' effectiveness for reducing risk factors for heart disease and stroke is limited.
A new study shows that walking and cycling for 20 minutes per day has greatest impact on lowering the risk of heart failure.
A sequential algorithm, which includes carotid sinus massage, tilt testing and implantable loop recorder implantation, helps to reduce syncope recurrence to 9% at one year in old patients with severe recurrent syncopes, according to results from the Syncope unit project 2 (SUP 2) study.
A seven-year study indicates the Stereotaxis remote magnetic navigation platform's success in ventricular tachycardia ablations compared to both contact force sensing and other manual catheters.
Study evaluates whether early detection of cardiac arrhythmias by BioMonitor with home monitoring reduces major cardiovascular events in post-acute myocardial infarction patients.
NICE Final Appraisal Determination recommends that edoxaban is a cost-effective use of NHS resources.
Deaths from heart attacks, strokes and other heart diseases have been declining, but social factors, including race, income, environment and education could reverse that trend.
BioCONTINUE is the first study to investigate the relevance of defibrillator back-up following first device replacement in a heart failure patient population with a primary indication for a cardiac resynchronisation therapy defibrillator (CRT-D).
Boston Scientific is also to become the exclusive global sales and marketing representative for the company's cardiology-related offerings.
The Atrial Fibrillation Network (AFNET) association and the European Society of Cardiology (ESC) are initiating a new trial (NOAH – AFNET 6) investigating whether patients without diagnosed atrial fibrillation who present with atrial high rate episodes (AHRE) benefit from...
The LuxCath LLC system determines electrode-tissue contact as well as monitors lesion progression during ablation and provides real-time lesion visualisation without pressure sensors or ultrasound.
A sensor-based electromagnetic tracking system helps to improve cardiac resynchronisation therapy (CRT) implantation facilitating speed of the procedure, reducing exposure to radiation and improving success rate of access to the coronary sinus, according to a study presented at EHRA EUROPACE - CARDIOSTIM (21-24 June, Milan, Italy).
The use of mobile smartphones and tablets that run applications (apps) to function as clinical examination tools, reference databases, technique guides, or medical calculators is today a reality in hospitals and healthcare centres; but what about their safety, accuracy...
A press release states that the company is now the only one offering both implantable cardioverter-defibrillators (ICDs) and pacemakers approved for 3T scans.
Key topics from the hot line sessions of ESC 2015 include atrial fibrillation, pacing, acute myocardial infarction, heart failure, hypertension, diabetes mellitus, pharmacology and coronary artery disease.
The study will be conducted in healthy volunteers. If Brinavess successfully completes phase 1, Eddingpharm anticipates initiating a pivotal phase 3 study by year end.
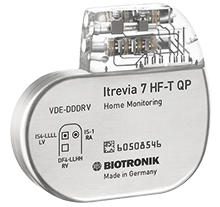
Itrevia HF-T QP includes the Closed Loop Stimulation (CLS) algorithm, capable of adapting heart rate in response to physiological demands independent of body movements or respiratory rate.
CardioMEMS (St Jude Medical), FDA approved in 2014, is a minimally invasive device used to measure the pulmonary artery pressures and heart rates of patients with NYHA Class III heart failure who have been hospitalised for heart failure in...
Black Americans are more likely to experience sudden cardiac arrest and at a much earlier age than their white compatriots, according to research published in Circulation.
A Food and Drug Administration (FDA) announcement and a study in JAMA Internal Medicine about use of the Lariat suture delivery device (SentreHeart) for left atrial appendage (LAA) closure have raised questions regarding using the device in this off-label indication.
A new study reports that within 12 months of completing anthracycline treatment, 57% of breast cancer patients had changes on their echocardiograms consistent with diastolic dysfunction.
Exposure to fine particle air pollution during wildfires may increase risk for cardiac arrest and other acute heart problems, particularly in the elderly, a new study has found.
Michael Glikson (director of Davidai Arrhythmia Center, Heart Center, Sheba Medical Center, Tel Hashomer, Israel), vice-chairperson scientific programme for EHRA EUROPACE-CARDIOSTIM 2015, talks to Cardiac Rhythm News about the highlights of this years' congress.
The US Food and Drug Administration has issued a safety communication to healthcare providers reporting deaths and serious adverse events with the use of the Lariat Suture Delivery Device (SentreHeart) and its associated devices used for left atrial appendage closure.
This video from the Organization for Occupational Radiation Safety in Interventional Fluoroscopy (ORSIF) tells the story of one of the world’s most prominent cardiovascular surgeons, Edward Diethrich, and the career-altering health issues he has faced as a result of chronic, low-level...
Novartis has announced that the US Food and Drug Administration (FDA) has approved Entresto (sacubitril/valsartan) tablets, previously known as LCZ696, for the treatment of heart failure with reduced ejection fraction.
The trial will evaluate the safety and effectiveness of the Sensei robotic system and Artisan family of catheters for introducing and positioning radiofrequency ablation catheters in patients with symptomatic, drug-refractory paroxysmal atrial fibrillation.
First patient enrolled in global clinical investigation into differences in gender response to cardiac resynchronisation therapy.
New study published in HeartRhythm shows differences in the utilisation of atrial fibrillation therapies in a large nationwide population.
Michael Gallimore, from the School of Engineering at the University of Lincoln, UK, and colleagues have created a new algorithm which produces more accurate electrocardiogram (ECG) signal classification when tested on patients.
Maria Grazia Bongiorni (Pisa, Italy) compares mechanical transvenous lead extraction vs. laser lead extraction approaches with supporting evidence from key randomised controlled trials in the field.
In the last few decades, the number of cardiovascular implantable electronic devices (CIEDs)...
An interim analysis of the phase III RE-VERSE AD patient study demonstrates that 5g of the antidote idarucizumab reversed the anticoagulant effect of dabigatran within minutes in patients with serious bleeding complications or requiring urgent procedures.
Sean D Pokorney (Durham, USA) and others report in JAMA that only 8.1% of older patients who are eligible to receive an implantable cardioverter defibrillator (ICD) after a myocardial infarction actually do so. The authors also found that older age did not appear to affect the mortality benefit that is associated with ICD implantation.
Until the closing of the transaction, expected in the third calender quarter of 2015, both companies will continue to operate separately under their current brand names and leadership structures.
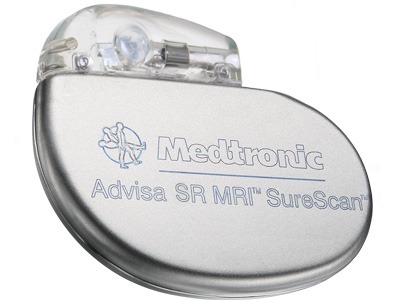
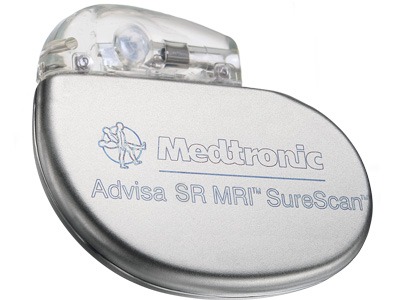
The Advisa SR MRI SureScan single-chamber pacemaker with the 5076 MRI lead allows for magnetic resonance imaging (MRI) scans positioned on any region of the body without restrictions.
The randomised, controlled AMAZE trial, will evaluate the use of the Lariat device for the ligation of the left atrial appendage as an adjunctive treatment to ablation in patients with persistent or long-standing persistent atrial fibrillation.
findings of a study demonstrate the promise in regenerating cardiac tissue using engineered patches made up of a mixture of fibrin and mesenchymal stem cells (MSCs) derived from human umbilical cord blood.
Edoxaban (Lixiana, Daiichi Sankyo) is an oral, once-daily selective factor Xa-inhibitor for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors.
The first ESC recommendations for patients with cardiac arrhythmias and chronic kidney disease (CKD) were presented at EHRA EUROPACE - CARDIOSTIM 2015 (21-24 June, Milan, Italy) and published in EP Europace.
The reduction in blood loss correlated with reversal of the anticoagulant effects of rivaroxaban as measured by anti-Factor Xa activity.
The recommendations are an update of EHRA's 2008 consensus document, which required an update due to dramatic changes in the field during the last five years.
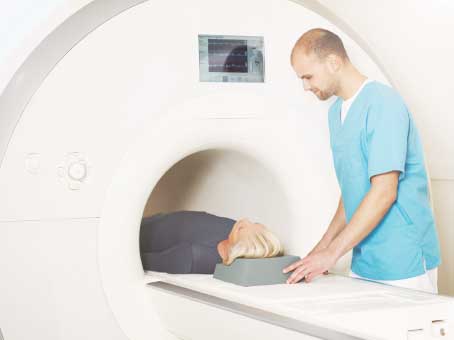
Patients are "test driving" a pacemaker outside the skin before deciding whether to have a permanent implant, according to research presented at EHRA EUROPACE-CARDIOSTIM by Michael Giudici, University of Iowa Hospitals and Clinics, USA.
The data from a global clinical trial involving the miniaturised device were presented at a late-breaking clinical trials session at EHRA EUROPACE-CARDIOSTIM 2015 (21-24 June, Milan, Italy).
The UNTOUCHED study will compare outcomes during an 18-month follow-up period to objective performance criteria derived from the MADIT-RIT study, which evaluated shock rates in 1,500 patients implanted with transvenous ICD devices.
Cecilia Linde speaks to Cardiac Rhythm News about her work on a research platform for new onset heart failure in Stockholm, the highlights of this year's EHRA EUROPACE - Cardiostim Congress and her views on what is needed to improve cardiac care in Europe.
The Confidense module's proprietary algorithm streamlines data collection, annotation and validation for the market-leading Carto system.
CardioInsight Technologies will now become part of the Medtronic Atrial Fibrillation Solutions business in the Cardiac Rhythm and Heart Failure division.
The AliveCor Mobile ECG and the AliveECG app allows users to detect the presence of atrial fibrillation in an electrocardiogram (ECG or EKG) and manage their heart health with an electrocardiogram ECG monitor.
Real-life data on stroke prevention from 17,200 patients will provide information on how patient risk profiles and quality of vitamin K antagonist control are associated with increased mortality and stroke in patients with newly diagnosed atrial fibrillation.
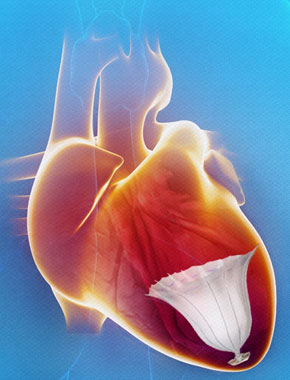
The publication of important new long-term data from nearly 900 patients and a rapid evolution in the technology is putting entirely the Subcutaneous Implantable Cardioverter Defibrillator (S-ICD, Boston Scientific) at the heart of a monumental shift in the utilisation...
Technologies to image lesion creation in real time are required to improve the efficacy as well as the safety of atrial fibrillation (AF) ablation. By improving efficacy of ablation, the requirement and attendant risks of repeat atrial fibrillation ablation...
In June 2015, cardiac rhythm specialists will gather in Milan, Italy, to learn, share and discuss the latest advances in cardiac arrhythmias treatment in the largest European congress in the field: EHRA EUROPACE-CARDIOSTIM.
The results from a retrospective study, based on a large dataset (over 140,000 patients), have cast doubt on the benefit of routine oral anticoagulants for patients with atrial fibrillation and low risk of stroke.
The Heart Journal is a feature that allows users to log and tag daily activities, symptoms and events in real-time that can impact heart health and work to identify abnormalities.
The InvisionHeart ECG system provides a mobile solution for capturing and managing 12-lead ECGs, including the ability to read and visually compare, confirm, report and store diagnostic quality electrocardiograms.
A study in healthy volunteers investigating the reversal of the anticoagulant effect of dabigatran etexilate (Pradaxa, Boehringer Ingelheim) by its specific agent idarucizumab has shown the antidote led to immediate, complete and sustained reversal of the anticoagulant effect.
Eighty AED Plus units will be distributed among the mobile medical teams and be available across all Baku 2015 venues.
The data from the American Heart Association will inform ongoing efforts and outreach about heart failure in the USA.
intravenous vernakalant facilitated successful electrical cardioversion in patients who had failed to attain sinus rhythm following failed electrical cardioversion, or who immediately returned to atrial fibrillation after briefly attaining sinus rhythm.
William Gold spoke to Cardiac Rhythm News about his involvement in various clinical trials, his views on the upcoming treatment options for heart failure and the highlights of this year's HRS meeting.
Report recommendations aim to improve management of atrial fibrillation as numbers are expected to double from 8.8 to 17.9 million adults aged over 55 years between 2010 and 2060.
The third-generation cryoballoon is designed to allow enhanced positioning and help improve capture of real-time data with achieve mapping catheter.
Long-term Citadel/Centurion clinical trial findings and independent data presented at Heart Rhythm Society 36th Annual Scientific Sessions.
Early performance results of the first-in-human international study of Medtronic's Micra Transcatheter Pacing System (TPS) have demonstrated the device is safe and effective.
This film stars cardiologist John C Grammer, who shows his novel way of demonstrating various heart rhythms and cardiac arrhythmias.
https://youtu.be/TJR2AfxVHsM
Early performance results of the first-in-human international study of Medtronic's Micra Transcatheter Pacing System (TPS) have demonstrated the device is safe and effective.
Armin Luik presented findings at HRS 2015 indicating that cryoballoon ablation is a faster as effective as radiofrequency ablation for pulmonary vein isolation in paroxysmal atrial fibrillation patients, but has a greater complication rate and requires higher X-ray dosages.
The study assesses the safety and efficacy of uninterrupted anticoagulation with dabigatran etexilate (Pradaxa) during ablation procedures compared to warfarin. Results from the study are expected during 2016.
Results from the prospective, non-randomised, multicentre SELECT-LV trial were presented at the Heart Rhythm Society 36th Annual scientific Sessions and demonstrated promising efficacy and safety data.
Botulinum toxin injected into epicardial fat pads during coronary artery bypass graft surgery reduced the incidence of postoperative atrial fibrillation compared with placebo, with substantial suppression persisting after one year, a pilot study has found.
A phase II study onbaroreflex activation therapy treatment for heart failure patients with a reduced ejection fraction has found greater benefits for patients without a cardiac resynchronisation therapy device than for patients with one.
In the largest study on the relationship between activity and survival in ICD patients, researchers analysed how active participants were in the first 30-60 days after implantation and then over time up to four years.
The trial, which randomised CardioFocus' HeartLight one-to-one versus the Biosense Webster Thermocool catheter, met both primary efficacy and safety endpoints and demonstrated a low learning curve for physicians.
The data was presented at the Heart Rhythm Society's 36th Annual Scientific Sessions (13-16 May, Boston, USA) and shows the systems benefits compared with standard voltage-based mapping in patients with atrial flutter.
All of the first 140 patients in the trial were successfully implanted with the Micra TPS. The data were presented at Heart Rhythm 2015 (13-16 May, Boston, USA).
St Jude Medical has received CE mark approval of expanded labelling for its Ellipse implantable cardioverter defibrillator and its Durata and Optisure defibrillation leads. It has received CE mark approval for its Assurity MRI and Endurity MRI pacemaker device families.
The agreement will bring real-time, patient-specific heart electrical activity data to cardiac electrophysiology labs around the world to speed up the diagnosis of the sources of atrial fibrillation and other heart rhythm disorders.
RIO 2 study evaluates safety and effectiveness of moving the insertion procedure from hospital to office setting.
Sacha Salzberg cardiovascular surgeon (Heart Clinic Hirslanden, Cardiac Surgery Unit, Zurich, Switzerland) comments on catheter-based and surgical approaches for left atrial appendage occlusion as treatment strategies for stroke prevention in atrial fibrillation (AF) patients. He calls for a heart...
A first-in-human study shows low level transcutaneous electrical vagus nerve stimulation, a completely non-invasive approach, suppresses paroxysmal atrial fibrillation.
Japan is the world's largest MRI market with more MRI scanners per capita than any other country: approximately 47 registered machines per one million people.
In the largest study to date to examine return to work after cardiac arrest, researchers studied 4,354 patients in Denmark who were employed before they suffered out-of-hospital cardiac arrests between 2001 and 2011.
New device allows for earlier therapy adjustments in heart patients with pacemakers, implantable cardioverter-defibrillators, cardiac resynchronisation therapy and BioMonitor devices.
The results of a prospective, randomised trial demonstrate that moderately strenuous aerobic exercise, performed at home, for a select group of implantable cardioverter defibrillator (ICD) recipients was highly beneficial at improving cardiovascular performance. Importantly, the exercise did not compromise safety.
Patients with two or three of the predictors had a sudden cardiac death risk that was 145-times greater than patients with normal levels on all three measures.
The PINNACLE Registry is a cardiological outpatient quality improvement registry, which collects data about patients in participating cardiology practices to help providers evaluate and improve their adherence to current guidelines.
The use of continuous positive airway pressure was associated with a significant reduction in the recurrence of atrial fibrillation in patients with obstructive sleep apnoea, according to a new analysis of data.
For the first time, the American Heart Association has issued recommendations for healthcare providers treating people older than 40 with congenital heart disease.
The S-ICD System was shown to convert more than 98% of heart arrhythmias that can lead to sudden death. These data are comparable to efficacy outcomes found in transvenous ICD (TV-ICD) clinical trials (95-99%).
New Biotronik DX implantable cardioverter defibrillators with longer battery life and 42 joules on first shock offers "maximum energy", while the Itrevia implantable cardioverter defibrillator series is also approved.
Investigators report data from first cohort in destination therapy at the 35th annual International Society for Heart and Lung Transplantation Meeting in Nice, France.
Safety and efficacy results comparing patients previously treated with cardiac resynchronisation therapy to patients without cardiac resynchronisation therapy will be presented by Michael Zile from the Medical University of South Carolina, USA.
Data support registration of Andexanet Alfa Bolus-Only and Bolus-Plus-Continuous-Infusion dosing regimens to reverse anticoagulant effect of Factor Xa Inhibitors.
The study, published in JACC: Clinical Electrophysiology, found that the benefits of participation may outweigh risks for children with heart conditions.
Daiichi Sankyo has announced that Swissmedic, the regulatory authority of Switzerland, has granted approval of Lixiana (edoxaban), an oral, once-daily selective factor Xa inhibitor, for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation and treatment of venous thromboembolism in deep vein thrombosis.
Fellowship provides newly-graduated cardiothoracic surgeons with a unique opportunity to be trained by nationally recognised experts in atrial fibrillation surgery.
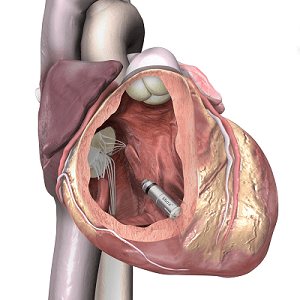
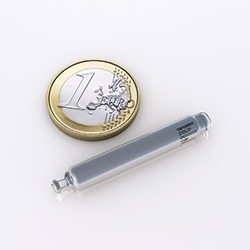
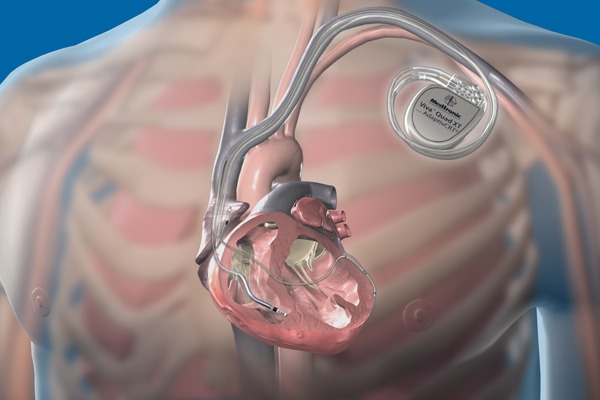
The device is less than one-tenth the size of traditional pacemakers and is delivered with minimally invasive techniques through a catheter, and implanted directly into the heart.
Portola Pharmaceuticals has announced positive topline results from the second part of the phase 3 ANNEXA-A study, which evaluated the safety and efficacy of andexanet alfa, an investigational antidote, with the Factor Xa inhibitor apixaban.
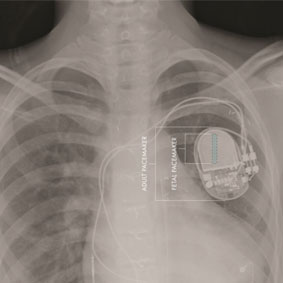
Researchers in the USA have developed the first fully-implantable micropacemaker designed for use in a foetus with complete heart block. The micropacemaker has been designated a humanitarian use device by the US Food and Drug Administration (FDA).
Speaking at the 2015 American College of Cardiology Annual Scientific Session, Luigi Di Biase reported that-compared with the antiarrhythmic drug amiodarone-catheter ablation significantly increases the rate of freedom of recurrence in patients with persistent atrial fibrillation and heart failure.
A new study has found that a combination of surgical atrial fibrillation ablation and mitral valve surgery in patients with persistent or long-standing persistent atrial fibrillation is associated with a significantly increased rate of freedom from atrial fibrillation at one year. However, it is also associated with a significantly increased rate of pacemaker implantation.
Several recent studies have validated that there is considerable mortality associated from a cardiac implantable electronic device infection in high-risk patients. A strategy using an antibacterial envelope is showing promising results not only reducing infection rates but also cutting...
Medtronic has announced the first patient enrolment in the GOLD AF registry, a first-of-its-kind, prospective, observational clinical study of its Phased Radiofrequency (RF) Ablation technology for treating patients with symptomatic atrial fibrillation.
A review of 1,247 sudden cardiac arrest cases involving men and women ages 35-65 revealed that 63 cases (5%) were associated mainly with sports activities.
Biotronik has announced that the first US patient has been implanted with the company's Eluna pacemaker system with ProMRI technology.
First-of-its-kind study published in HeartRhythm assesses various factors that lead to inaccurate detection of the frequency and duration of atrial fibrillation episodes.
The first study investigating the long-term effects of weight loss and the degree of weight fluctuation on atrial fibrillation (AF) burden has found that obese patients with this arrhythmia who lost at least 10% of their body weight were six times more likely to achieve long-term freedom from atrial fibrillation.
An interview with Martin Cowie
Martin Cowie (Imperial College London, London, UK) is the principal investigator of the SERVE-HF study, which is assessing the use of adaptive servo-ventilation (PaceWave, ResMed) in chronic heart failure patients with central sleep apnoea. In...
Biotronik's ProMRI technology allows patients to undergo full-body magnetic resonance imaging (MRI) scans with both single-chamber and dual-chamber Eluna pacemakers when implanted with Setrox pacing leads.
CorMatrix announced that it has received international approval from Argentina's National Administration of Drugs, Foods and Medical Devices (ANMAT) to market the CorMatrix CanGaroo ECM Envelope. canga
The Emblem S-ICD system is 19% thinner and is projected to last 40% longer than the previous system, improving patient comfort and cosmetic outcomes while reducing the number of times the device will require replacement.
William M Bailey, medical director of Louisiana Heart Rhythm Specialists in Lafayette, discussed new clinical data at the American College of Cardiology's 64th Annual Scientific Sessions in San Diego.
The GENETIC-AF phase 2B/3 clinical trial is evaluating Gencaro (bucindolol hydrochloride) as a potential treatment for atrial fibrillation.
Cleveland Heart International to acquire majority interest in Korean automatic external defibrillators manufacturer CU Medical.
First phase II data demonstrating anticoagulant prescribing patterns in North America now available from GLORIA-AF Registry programme.
The Society of Pediatric Cardiology Training Program Directors, the American College of Cardiology, the American Academy of Pediatrics and the American Heart Association worked to develop the guidelines.
This latest version defines cardiology core competencies for the first time.
Data from the two studies will be presented at the 64th Annual Scientific Sessions of the American College of Cardiology (ACC) (14-16 March, San Diego, USA).
Albert Hagège (Georges Pompidou European Hospital in Paris, France), principal investigator of Vanguard, a new study exploring the safety and efficacy of vagal nerve stimulation in heart failure patients with the Equilia system (Sorin Group), explains the details of...
First in-man multicentre study of the miniaturised implantable cardiac monitor Reveal Linq (Medtronic) suggests the device is safe and provides adequate sensing capabilities to detect arrhythmias.
TomTec-Arena is a suite of clinical applications to review, analyse and quantify medical image data in multiple dimensions (2D and 3D/4D) and multiple modalities.
The ResQCPR system demonstrated a 49% increase in one-year survival in adult patients who experienced out-of-hospital cardiac arrest of presumed cardiac etiology, as compared to treatment with conventional manual CPR.
Anytime an ECG is taken with the AliveCor Heart Monitor, the AliveECG app will tell users if atrial fibrillation is present, if the ECG is normal, or if there is too much interference and another ECG should be taken.
Analysis of 19 studies finds atrial fibrillation patients taking digoxin are 27% more likely to die than those who do not.
Two-thirds of patients who were not previously recommended for oral anticoagulation therapy are now recommended, according to an assessment of the 2014 AHA, ACC and HRS atrial fibrillation guidelines.
Alec Momont (graduate student, Technical University of Delft IDE, The Netherlands) has designed an ambulance-drone capable to deliver defibrillation to cardiac arrest victims in a 12 km2 area within one minute. "At that speed, survival rates can be as...
Boehringer Ingelheim has announced it has submitted a biologics license application to the FDA, requesting an Accelerated Approval pathway, for the use of idarucizumab to reverse the anticoagulant effect of dabigatran.
Quail's wireless headset system will provide hands-free communications between the treatment lab and monitoring room, which are separated by insulated partitions and glass.
The market will more than double in value from US$4.6bn in 2013 to US$9.4bn in 2020, before declining to US$5.7bn by 2023, according to research and consulting firm GlobalData.
Sorin and Cyberonics have announced their merger plan with a combined equity value of approximately US$2.7bn (€2.4bn).
The risk is higher for older women, unmarried people and those with chronic conditions that affect mobility and ability, including obesity, dementia, anaemia and diabetes, researchers said.
Subcutaneous implantable cardioverter-defibrillator patients may be safe to be exposed to magnetic resonance imaging (MRI) procedures at 1.5T with a pre-specified scanning and monitoring protocol.
Vitaria delivers autonomic regulation therapy for patients who have moderate to severe heart failure with left ventricular dysfunction (ejection fraction < 40%), and who remain symptomatic despite stable, optimal heart failure drug therapy.
Results of a prospective, multicentre pilot trial of severe heart failure patients treated with continuous spinal cord stimulation have shown the treatment is safe and feasible with the Eon Mini neurostimulation device (St Jude Medical) and can potentially improve symptoms, functional capacity and cardiac function.
The study is the first-of-its-kind in the USA exploring a minimally invasive epicardial surgical ablation approach combined with an endocardial catheter-based ablation approach for the treatment of persistent or long-standing persistent atrial fibrillation.
The Stronger Hearts Helpline pilot programme centralises resources to educate and improve access to care.
The study, which was funded by iRhythm, suggests that the lack of diagnosis after the initial use of the Holter monitor resulted over US$23,000 per patient in wasted spending by Medicare.
With the launch, the hospital becomes the first in Europe to operate two Niobe systems for clinical procedures.
Studies validate safety of Biotronik ProMRI devices in patients subjected to head and lower lumbar 1.5T MRI scans.
The Vanguard (Vagal nerve stimulation safeguarding heart failure patients) clinical study evaluates Equilia, a neurostimulation system for heart failure patients.
A new study has examined the therapeutic effects of PDA-001, an intravenous formulation of PDAC cells, in mice, as well as the best way to deliver the therapy.
New data demonstrate cost-effectiveness of long-term, continuous cardiac monitoring with potential to prevent recurrent strokes.
The largest study using the Amplatzer Cardiac Plug (St Jude Medical) for left atrial appendage occlusion has shown a high procedural success rate and favourable outcomes for prevention of atrial fibrillation-related thromboembolism.
Results of the X-VERT trial, presented at the European Society of Cardiology meeting (ESC; 30 August–3 September, Barcelona, Spain), showed that rivaroxaban (Bayer/Janssen Pharmaceuticals) appears to be an effective and safe alternative to vitamin K antagonist (VKA) therapy in...
Savaysa (edoxaban) is an oral, once-daily selective factor Xa inhibitor, designed to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation
The amended protocol now includes the use of the Thermocool SmartTouch catheter (BioSense Webster) and the EnSite Velocity Cardiac Mapping System (St Jude Medical) in combination with the Sensei Robotic System (Hansen Medical) for the treatment of paroxysmal atrial fibrillation.
Andexanet alfa is the only universal factor Xa inhibitor antidote shown to directly reverse the anticoagulant activity of these agents in clinical studies.
The new sensor is as accurate as the "wet electrode" sensors used in hospitals, but can be used for long-term monitoring and is more accurate than existing sensors when a patient is moving.
CVRx completed enrolment of the 146-patient clinical trial to determine the performance of Barostim Therapy for patients suffering from chronic heart failure with advanced symptoms.
The devices feature EnduraLife battery technology developed with high-performance chemistry and advanced manufacturing capabilities to provide up to double the battery capacity of other defibrillators.
While Hung-Fat Tse's initial research interests focused on device therapy for cardiac arrhythmias, he has dedicated his career to the development of cardiovascular regenerative medicine.
A subanalysis from the ORBIT-AF registry has found that atrial fibrillation patients who receive bridging anticoagulation therapy during anticoagulation interruption are at higher risk of bleeding and adverse events following interruption than patients who do not receive bridging anticoagulation.
AliveCor's new algorithms include a Normal Detector that identifies when no abnormalities are detected in an ECG recording and an Interference Detector that identifies if factors have affected the recording making the ECG unreadable.
In addition, Xarelto (rivaroxaban) label now includes guidance for use in patients with atrial fibrillation undergoing cardioversion.
The FlexAbility catheter features a handle and shaft combination that allows for improved maneuverability, enabling electrophysiologists to reach challenging anatomic locations within their patients' hearts.
Guideline adherence to anticoagulation in atrial fibrillation seems to be more difficult compared to other quality measures in cardiology practice. William Lewis (chair of the Get With The Guidelines-AFIB Clinical Work Group) explains how the Get With The Guidelines-AFIB,...
The ProMRI study consists of a series of MRI compatibility trials intended to increase cardiac device patients' access to MRI.
Accessing data from the National Cardiovascular Disease Registry PINNACLE Registry, researchers examined a nationwide sample of 68,808 patients receiving aspirin for primary cardiovascular disease prevention.
The latest version of the AliveECG app for users in the United Kingdom and Ireland now provides patients with real-time atrial fibrillation detection in electrocardiogram recordings using the AliveCor Heart Monitor.
The trial design cleared by FDA is a multiple arm phase 2, multicentre, placebo-controlled study of intranasal administration of MSP-2017 for the conversion of PSVT to sinus rhythm.
In paroxysmal atrial fibrillation, pulmonary vein isolation is successful while the results in persistent atrial fibrillation are less satisfactory because of ill-defined atrial substrate including multiple atrial wavelets, macro-re-entries and sources. We have recently published a study in Circulation,...
The FlexAbility ablation catheter will be used to assess benefits of catheter ablation as an adjunctive therapy for patients at risk for life-threatening arrhythmias.
Cryoablation creates lesions by freezing tissue in the heart's upper chambers, traditionally around the pulmonary veins, to block the electrical signals that trigger erratic heart rhythms.
The trial will evaluate the effectiveness of the Tyrx absorbable antibacterial envelope in reducing major infections in patients with cardiac implantable electronic devices at risk of infection.
The Vdrive allows physicians to remotely control the advancement, retraction and rotation of a compatible fixed curve transseptal sheath, in conjunction with a magnetic ablation catheter.
Implementation of a screening programme of all competitive athletes to avoid sports related sudden cardiac death is one of the hottest topics in sports cardiology. Researchers in Denmark have found new data that questions this practice, Bjarke Risgaard and...
Abbott has acquired all outstanding equity of Topera for US$250m upfront, plus potential future payments tied to performance milestones.
The Biosense Webster Advantage programme is an outcomes-based, risk-sharing program for US hospitals that reinforces the significant patient benefits demonstrated by the ThermoCool SmartTouch Catheter.
Philips secures nationwide Department for Education tender to provide schools with the latest HeartStart FRx automated external defibrillators.
AliveCor says that the third generation device is 50% thinner and 40% lighter and was designed from first-hand customer feedback and in collaboration with global design firm IDEO.
Analysis of Biotronik-sponsored REPLACE registry identifies key patient risk factors and reveals comorbidities more relevant than procedure-related complications.
Preliminary data from the AFACART study have shown encouraging results when treating persistent atrial fibrillation with non-invasive mapping followed by pulmonary vein isolation and/or linear lesions.
George H Crossley (Nashville, USA) writes on the role of quadripolar leads in cardiac resynchronisation therapy to treat heart failure patients.
The American College of Cardiology's recently-released Guideline Clinical App has expanded with the addition of tools and guideline content for valvular heart disease and atrial fibrillation.
The first patient has been enrolled in the RE-SPECT ESUS phase III study to investigate the efficacy and safety of dabigatran etexilate for the prevention of recurrent embolic stroke of undetermined source.
The newest additions to the Attain Performa lead portfolio are designed to accommodate patients' varying vessel sizes and curvatures to enhance successful lead placement.
The Allure Quadra MP cardiac resynchronisation therapy pacemaker contains MultiPoint Pacing technology, which enables physicians to pace multiple locations on the left ventricle, giving the clinician more choices to best optimise CRT pacing based on patient need and reducing the rate of CRT non-responders.
Phase B of Biotronik's ProMRI study has completed patient enrolment and concluded all planned scans.
A new study evaluating Optim-insulated implantable cardioverter defibrillator leads found low rates of all-cause mechanical failure during a median follow-up of 3.2 years.
iRhythm Technologies has secured a CE mark for the ZIO Service, enabling the company to market its long-term continuous heart monitoring solution in Europe.
Biotronik has launched its new series of single- and dual-chamber implantable cardioverter defibrillators and cardiac resynchronisation therapy defibrillators.
For patients in cardiac arrest, administering epinephrine may increase the overall likelihood of death or debilitating brain damage, according to a study published in the Journal of the American College of Cardiology.
A new survey has found that despite an increase in the number of non-valvular atrial fibrillation patients compared to five years ago, the majority of cardiologists believe there is a delay in patients reaching diagnosis.
A study undertaken in the UK has shown that radiofrequency ablation of scar-related ventricular tachycardia, using robotic navigation, is safe and feasible and has resulted in 95% reduction in total implantable cardioverter defibrillator (ICD) therapy burden at six months.
Mikhail Kosiborod, of Saint Luke's Mid America Heart Institute, Kansas City, and colleagues evaluated the efficacy and safety of the drug zirconium cyclosilicate in patients with hyperkalemia (higher than normal potassium levels).
New data on the investigational antidote idarucizumab show that it may reverse the effect of the oral anticoagulant dabigatran (Pradaxa, Boehringer Ingelheim) on both blood coagulation and the blood clotting mechanism.
Jared Bunch (Murray, USA) speaks, at AHA 2014, about a new study, which shows how AF patients treated with a combination of antiplatelet and anticoagulant therapy may be at an increased risk of dementia.
Video courtesy of American Heart Association.
https://youtu.be/tFTBTmQUj94
Biotronik has launched ProMRI SystemCheck, a new online tool for tracking the ProMRI status of implantable devices.
Atrial fibrillation patients who are receiving a combination of antiplatelet and anticoagulant therapy and are over treated with warfarin may be at an increased risk of dementia, according to a study presented at the American Heart Association's Scientific Sessions (AHA;15-19 November, Chicago, USA).
Michel Haissaguerre and Etienne M Aliot, chairmen of the International Symposium on Catheter Ablation Techniques (ISCAT; 15-17 October 2014, Paris, France), discuss the highlights of this year's meeting with Cardiac Rhythm News.
New data from Medtronic show that its cardiac resynchronisation therapy devices for the treatment of heart failure can cause a significant reduction in all-cause 30-day readmissions after heart failure hospitalisations.
Retrospective data analysis from the CHAMPION clinical trial shows significant reduction in 30-day hospital readmission rates for heart failure patients age 65 and older treated with the CardioMEMS HF system (St Jude Medical).
LoneStar Heart has presented the primary six month results of its multicentre, randomised clinical trial of Algisyl-LVR, providing evidence that the cardiac hydrogel implant can prevent or reverse the symptoms of moderate to severe heart failure in patients with a dilated and weakened left ventricle.
New data from PARADIGM-HF have shown that the investigational angiotensin receptor-neprilysin inhibitor (ARNI) LCZ696 (Novartis) has the potential to prevent the clinical progression of surviving patients with heart failure more effectively than enalapril.
Stressful and physically-demanding law enforcement activities are associated with large increases in the risk of sudden cardiac death among US police officers compared with routine policing activities.
A survey issued by the Heart Rhythm Society and National Stroke Association in collaboration with Boehringer Ingelheim has found there are information gaps regarding the impact of AF-related stroke - among AF patients, physicians and caregivers - including communication barriers, challenges with patient education, misperceptions about treatment compliance, and outcomes related to the impact of stroke on one's life.
A study has investigated the clinical significance of repeat testing after puberty in asymptomatic children with a family history of Brugada syndrome who had an initial negative test earlier in childhood.
Study examines the long-term efficacy and safety of Boston Scientific's Watchman device to achieve left atrial appendage closure in patients with atrial fibrillation.
Biosense Webster has partnered with advocacy organisation StopAfib.org to encourage Americans to "Get SMART About Afib."
The American College of Cardiology has announced the launch of the Journal of the American College of Cardiology (JACC): Clinical Electrophysiology, which will feature original research and review articles regarding cardiac rhythm disorders.
Medtronic has announced the results of a new study which found that atrial fibrillation and bradycardia occurred at higher than expected, and clinically significant, rates in patients with end-stage renal disease undergoing haemodialysis.
Boston Scientific has announced that its subcutaneous implantable defibrillator (S-ICD System) will have designated Current Procedural Terminology (CPT) Category I codes by the American Medical Association, effective 1 January 2015.
Researchers in Switzerland are developing a pacemaker which has no leads and no battery. With this in mind, the prototype is aimed to solve two of the major problems with traditional pacemakers. The idea of the device was inspired...
Online electrocardiogram (ECG) continuous surveillance during marathon running is feasible, according to a study presented at the European Congress on e-Cardiology and e-Health (29-31 October, Bern, Switzerland). The proof-of-concept study may allow "instantaneous diagnosis of potentially fatal rhythm disorders" in endurance athletes.
Dominic Abrams (Division of Cardiac Electrophysiology, Boston Children's Hospital, Boston, USA) writes on the importance of arrhythmia management in congenital heart disease patients and identifies the reasons why it remains a major challenge for interventional eletrophysiology.
The study achieved the primary endpoint, showing that 53% of patients with recent onset atrial fibrillation (lasting three hours to seven days) receiving an intravenous dose of vernakalant converted to normal heart rhythm within 90 minutes, compared to 12% of placebo patients.
The American College of Cardiology (ACA), which has been developing clinical practice guidelines in partnership with the American Heart Association (AHA) for more than three decades, has launched a new Guideline Clinical App to serve as the mobile home for all ACC/AHA guideline content and related tools.
The DEEP study will investigate a minimally invasive approach performed by cardiac surgeons and electrophysiologists to treat patients with persistent or long-standing persistent atrial fibrillation who have failed antiarrhythmic drug therapy.
LifeWatch AG has signed an agreement with Vital Connect Inc. to utilise its HealthPatch MD as a 1-lead ECG device in its cardiac monitoring business.
A study of Medicare beneficiaries suggests that dabigatran (Pradaxa, Boehringer Ingelheim) should be prescribed with caution, especially among high risk patients, as it may be associated with a higher incidence of major bleeding and a higher risk of gastrointestinal bleeding, despite a lower risk of intracranial haemorrhage than warfarin, according to a study published online in JAMA Internal Medicine.
Hansen Medical's Sensei X2 robotic system is now commercially available in the USA and European Union.
Daiichi Sankyo has announced that the FDA has recommended approval of once-daily Savaysa (edoxaban) 60mg dosing regimen.
A study has found that patients with sinus bradycardia during therapeutic hypothermia had a 50-60% lower mortality rate at 180 days than those with no sinus bradycardia.
Vitamin D deficiency increases the risk of poor brain function after sudden cardiac arrest by seven-fold, according to research presented at Acute Cardiovascular Care 2014 by Jin Wi.
St Jude Medical has announced the US Food and Drug Administration (FDA) approval of its TactiCath Quartz irrigated ablation catheter, the company's newest technology that gives physicians a real-time, objective measure of the force that the catheter applies to a patient's heart wall during an ablation procedure.
Abbott enters the catheter-based electrophysiology market with the announcement of an agreement to purchase Topera and the securement of the rights to purchase Advanced Cardiac Therapeutics (ACT). Both companies develop electrophysiology technologies for the diagnosis and treatment of heart rhythm disorders.
An international multicentre registry evaluating the role of remote robotic navigation and contact force sensing in persistent atrial fibrillation (AF) treatment has found that, at one year, a higher proportion of patients treated with this modality were free from AF recurrences with a single procedure as compared with both manual/contact force sensing and robotic/no contact force sensing procedures.
A new study has found that living close to a major road may increase women's risk of dying from sudden cardiac death.
Boston Scientific has announced the CE mark approval for the Accolade pacemaker family and for the Visionist and Valitude cardiac resynchronisation therapy pacemakers (CRT-P) with quadripolar pacing technology.
Medtronic has announced the US Food and Drug Administration (FDA) approval of its CapSureFix Novus MRI SureScan 5076 lead for use with magnetic resonance imaging (MRI).
The Massachusetts General Hospital Institute for Heart, Vascular and Stroke Care has announced participation as coordinating centre in the USA for a new research initiative dedicated to explore the genetic causes of atrial fibrillation as part of the Transatlantic Network of Excellence in Cardiovascular and Neurovascular Research Program.
AtriCure has announced that enrolment in the ABLATE post approval study (PAS) of its Synergy Ablation System is complete. As 3 October 2014, the ABLATE PAS enrolled 365 patients at 40 hospitals across the United States.
Richard Schilling (professor of Cardiology and Electrophysiology, Barts Health NHS Trust and Queen Mary University of London, UK) speaks about the use of robotic navigation and catheter force sensing technology for atrial fibrillation treatment at Barts.
https://youtu.be/-P9XmeiGXuQ
Members of the US Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee have voted in favour of the Watchman left atrial appendage closure (LAAC) device (Boston Scientific), by a close vote of 6 to 5 (with 1 abstention) concluding that the potential benefits of Watchman outweigh the potential risks. The Panel also voted unanimously in favour of the device safety; however, its effectiveness saw unfavourable voting.
Massimo Santini performed the first fulguration of the atrioventricular node of resistant supraventricular tachycardia in Italy; he also implanted a pacemaker in the Italian president Carlo Azeglio Ciampi, 10 years ago. Santini speaks to Cardiac Rhythm News about his views on advances in antiarrhythmic drugs, genetics and cardiac arrhythmias, and ECG screening.
Cardiovascular scientists in the USA have identified in mouse models a protein known as Purkinje cell protein-4 (Pcp4) as a regulator of the heart's rhythm. Additionally, they have found that when the Pcp4 gene is disrupted, it can cause ventricular arrhythmias.
CVRx has announced the first 10 heart failure patients treated with the Barostim neo system under CE mark approval.
Randy Lieberman (Detroit, USA) explains how lead design for vagal nerve stimulation in heart failure treatment may affect the outcome of the procedures.
Vagal nerve stimulation has shown improvements in heart failure using several devices in many animal studies...
Before undergoing heart imaging procedures involving radiation, healthcare providers should help patients understand why the procedure is needed and its potential benefits and risks, including risks related to radiation exposure, according to a new scientific statement in the American Heart Association's journal Circulation.
The American College of Cardiology, along with eight partnering societies, have released the first appropriate use criteria for suspected heart disease in paediatric patients.
Reflecting on the World Heart Day, spokespersons from ESC call for the need to encourage cardiovascular disease prevention through healthy diets and increment of physical activity.
Atricure has announced the introduction of the AtriClip Flex, a new device used in left atrial appendage occlusion for stroke prevention in atrial fibrillation patients. According to a company release, the device has a more flexible aluminum shaft that allows surgeons to better manoeuvre within a patient's particular anatomy.
St Jude Medical has announced CE mark approval of updated labelling for its Tendril STS and IsoFlex Optim pacing leads. The new labelling allows existing and future patients with the devices access to magnetic resonance imaging (MRI) scans.
Medtronic has announced the US launch of the Seeq Mobile Cardiac Telemetry (MCT) System, an external, wire-free, adhesive heart monitor that can be worn for up to 30 days to help detect and diagnose the cause of irregular heartbeats.
St Jude Medical has announced a new data analysis from the CHAMPION clinical trial-in a subgroup of patients with renal failure-that showed reduced heart failure hospitalisation by 42% for patients managed with pulmonary artery pressure based technology compared to a control group.
The American Heart Association (AHA) and American College of Cardiology (ACA) have released a statement including 14 key elements that can be used as a checklist for screening young people age 12-25 for congenital and genetic heart disease.
NECTAR-HF, the first randomised, sham-controlled trial of vagal nerve stimulation for the management of heart failure has failed to show significant difference in cardiac remodelling between vagal nerve stimulation and a sham procedure-meaning that the study has not met its pre-specified six-month primary efficacy endpoint.
e-Health encompasses the use of information and communication technologies in support of health and health-related activities, including healthcare services, health surveillance, education, knowledge management, analytics, and research. Nico Bruining, Enrico G Caiani, Catherine E Chronaki, Przemyslaw Guzik and Enno...
Results from the X-VERT trial have shown that rivaroxaban is a safe and effective alternative to Vitamin K antagonist (VKA) therapy in patients with atrial fibrillation who are undergoing elective cardioversion.
Preventice, developer of the PatientCare Platform and the BodyGuardian Remote Monitoring Sensor and eCardio, a provider of remote cardiac monitoring products and services, have announced a strategic combination to drive innovation and growth in remote monitoring systems and mobile health applications.
Uncertainty exists about the optimal post-interventional antithrombotic drug regimen as well as treatment duration after left atrial appendage closure; Boris Schmidt and KR Julian Chun review different strategies. Schmidt spoke on the subject at Cardiostim/EHRA Europace (Nice, France, 18–21...
A new sudden death predictor for patients with hypertrophic cardiomyopathy identifies candidates for implantable cardioverter defibrillators (ICDs) in ESC Guidelines published recently. They were presented at the European Society of Cardiology (ESC) Congress by Task Force chairperson Perry Elliott (The Heart Hospital, London, UK).
Permanent atrial fibrillation (AF) doubles the risk of stroke compared to paroxysmal AF, according to research in more than 6,000 patients presented at European Society of Cardiology Congress. The findings suggest that a simple clinical assessment of the type of AF can help doctors to better estimate stroke risk.
Results from the EuroEco trial have found that the cost of remote monitoring (with Biotronik Home Monitoring) of patients with implantable cardioverter defibrillators (ICDs)-to physicians, hospitals and insurance providers-does not differ significantly from traditional in-office monitoring.
Findings from the PARADIGM-HF trial have found that the investigational medicine LCZ696 (Novartis) cuts cardiovascular deaths by 20% compared to ACE inhibitor enalapril in heart failure patients with reduced ejection fraction.
Stereotaxis has announced that it has received 510(k) clearance by the Food and Drug Administration (FDA) to market its Vdrive robotic navigation system with V-Loop variable loop catheter manipulator in the USA.
New data indicate that kidney function decline is less pronounced in patients with non-valvular atrial fibrillation who are treated with Pradaxa (dabigatran etexilate) compared to warfarin.
During this final phase of the registry programme, data on the overall safety and effectiveness of antithrombotic treatments will be collected.
The IN-TIME trial, which was recently published in The Lancet, demonstrated that heart failure patients' mortality can be reduced by more than 50% using Biotronik Home Monitoring.
BioControl Medical has announced that it has reached 480 randomised patients - or 70% - of the planned 650 subjects with congestive heart failure in the INOVATE-HF trial of its CardioFit system.
Results from an analysis of the German Statutory Insurance database showed that the three-year mortality of people with sleep apnoea was significantly lower in patients who were treated with positive airway pressure devices compared to a similar cohort who were not.
St Jude Medical has announced results from a large-scale, clinical study concluding that the Quartet left-ventricular quadripolar lead provides more options to effectively manage common pacing complications compared to systems with bipolar leads.
Néstor López Cabanillas and Elencwajg Benjamin from Buenos Aires, Argentina, write about two similar novel techniques for cardiac resynchronisation therapy (CRT) implantation, currently used in some countries in Latin America and Europe, which are showing favourable results in heart...
Results from the SMART-AF trial, recently published in the Journal of the American College of Cardiology, have shown that the ThermoCool SmartTouch contact force-sensing catheter is safe and effective for the treatment of drug-refractory symptomatic paroxysmal atrial fibrillation.
Medtronic has announced the first implants in a clinical trial that will compare patient and healthcare system outcomes in heart failure patients who have cardiac resynchronisation therapy (CRT) devices with the AdaptivCRT feature enabled versus patients receiving standard CRT.
CorMatrix Cardiovascular has announced that it has FDA clearance to market the CorMatrix CanGaroo ECM envelope for use with cardiac implantable electronic devices, including pacemakers and implantable cardioverter defibrillators (ICD's).
A new European joint consensus document on the use of antithrombotic drugs in patients with atrial fibrillation presenting with an acute coronary syndrome and undergoing percutaneous coronary intervention has been published in the European Heart Journal.
A recent feasibility study has found irrigated multi-electrode radiofrequency ablation of atrial fibrillation using the novel 10-pole circular, open-irrigated mapping and ablation catheter nMARQ device (Biosense Webster) to be fast and effective.
Medtronic has announced the US Food and Drug Administration (FDA) approval of its newest cardiac resynchronisation therapy-pacemaker, Viva CRT-P, for indicated patients with heart failure or atrioventricular block.
Impulse Dynamics has announced the first implantation of the Optimizer IVs device in the Czech Republic earlier this summer. The implantation took place at the Na Homolce Hospital in Prague (Czech Republic) by Petr Neuzil, head of Cardiology at the same institution.
John Muir Health's Concord medical centre is one of just 20 hospitals in the USA to begin treating patients who suffer from atrial fibrillation with a new procedure that features the FDA-cleared FIRMap catheter.
Bristol-Myers Squibb Company and Pfizer have announced that they will present 14 abstracts (oral and poster presentations) at the ESC Congress 2014, 30 August to 4 September in Barcelona, Spain.
AliveCor has announced that the US Food and Drug Administration (FDA) has granted the company clearance for its algorithm to detect atrial fibrillation.
Increasing the amount or intensity of physical activity can cut the chances of older women developing atrial fibrillation, according to new research in the Journal of the American Heart Association (JAHA).
New data revealing the reduction in cardiovascular deaths with Novartis' LCZ696 in patients with heart failure with reduced ejection fraction will be presented at the European Society of Cardiology (ESC) Congress 2014.
Hansen Medical and Adachi have announced a distribution agreement for Magellan and Sensei Robotic Systems in Japan. As part of the distribution agreement, Adachi is responsible for obtaining Japanese Marketing approval from the Pharmaceuticals and Medical Devices Agency for the systems.
Dhanunjaya Lakkireddy, director, Center for Excellence in AF and Complex Arrhythmias, University of Kansas Medical Center, Kansas City, USA, explores the effects of yoga in atrial fibrillation patients, including whether yoga can delay the transition from paroxysmal atrial fibrillation...
A first US multicentre registry with the Lariat device (SentreHeart) for left atrial appendage ligation has shown 94% acute closure rate; however, procedural success has been limited with major bleeding.
Biotronik has announced that the IN-TIME study results have been published in The Lancet. The randomised, controlled trial is the first worldwide to demonstrate that automatic, implant-based remote monitoring leads to significant clinical benefits for heart failure patients.
After over 10 years of research, scientists in the United States have demonstrated—for the first time—the feasibility of a somatic cell reprogramming strategy for creating a biological pacemaker in a large animal preclinical model. Eugenio Cingolani, assistant professor of...
Elizabeth Saarel (University of Utah, Salt Lake City, USA) argues that, based on prospective registry data, young patients with implantable cardioverter defibrillators can participate safely in competitive sports.
Medtronic has received US Food and Drug Administration (FDA) approval for the Attain Performa Model 4298 quadripolar lead, and the Viva Quad XT and Viva Quad S cardiac resynchronisation therapy defibrillators (CRT-D).
St Jude Medical has announced that the Center for Medicare and Medicaid Services (CMS) has approved a New Technology Add-on Payment (NTAP) for the CardioMEMS heart failure system.
Cardiome Pharma has announced submission of the Brinavess reimbursement dossier to Belgium's national compulsory health care and benefits insurance authority, The National Institute for Health and Disability Insurance.
Marking the 30th anniversary of the publication of their first joint guidelines for the diagnosis and treatment of heart disease, the American College of Cardiology and the American Heart Association have published an extensive review of the process and methodology for evaluating cardiovascular research and writing practice guidelines for clinicians.
A first, large multicentre study in the United States has found that despite the advances in clinical management strategies after heart transplantation, there has not been an improvement in the incidence of sudden cardiac death (SCD) in heart transplant patients over the last three decades.
Dierk Thomas (Heidelberg, Germany) writes on the results of preclinical research on antiarrhythmic gene therapy.
Imaging of the heart has played an important role in defining cardiac structures and characterising the arrhythmic substrates. Among the available imaging modalities, magnetic resonance imaging (MRI) has led to a better understanding of the mechanisms of cardiac arrhythmias...
Running for only a few minutes a day or at slow speeds may significantly reduce a person's risk of death from cardiovascular disease compared to someone who does not run, according to a study published in the Journal of the American College of Cardiology.
Cardioxyl Pharmaceuticals has announced the positive results of a clinical trial demonstrating that CXL-1427, a novel potential treatment for acute decompensated heart failure (ADHF), was well tolerated in healthy volunteers.
Effective immediately, Cook Medical customers in the United States will again have access to its Evolution RL and Evolution Shortie RL controlled-rotation dilator sheath sets.
The device has been designed to reduce complications associated with ablation procedures through its ability to bend and conform to the cardiac anatomy, decreasing the amount of pressure distributed to a patient's heart wall while simultaneously increasing the stability of therapy delivery.
The three-day event is the first large-scale opportunity for the Stereotaxis' in-country distributors to promote the clinical value of the Niobe magnetic navigation system for cardiac ablations to the Japan electrophysiology market.
Even in moderation, consumption of wine and hard liquor may be a risk factor for atrial fibrillation, according to new research recently published in the Journal of the American College of Cardiology. The research did not identify a similar risk for moderate consumption of beer.
A low sense of self-competence seems to contribute to decreased quality of life for paediatric patients with pacemakers, according to results of a study recently published in the Journal of Developmental & Behavioral Pediatrics.
With Sentus quadripolar leads and the Inventra series, Biotronik offers the only devices for patients with heart failure worldwide that are approved for MRI use.
Nick Linker is president-elect of the British Heart Rhythm Society (BHRS) and programme director of Heart Rhythm Congress (HRC). He tells Cardiac Rhythm News about his leadership in the re-design of the BHRS certification process, his goals for his presidential tenure, the key aspects of running a successful electrophysiology department and the highlights of this year's HRC.
John D Day, president-elect of the Heart Rhythm Society writes that many cases of atrial fibrillation in the USA could be prevented or reversed with lifestyles focussed on health.
The American Heart Association/American Stroke Association's Get With the Guidelines quality improvement programme reached two milestones this month, touching the lives of more than five million patients, including more than three million people who had strokes.
Biotronik has announced that the first device patients to undergo full-body magnetic resonance imaging (MRI) scans in the United States have been implanted with the Biotronik DX system.
GlobalMed introduces the TotalECG, a battery-powered device that streams 12-Lead data securely and wirelessly to interpretation software on a computer that could be up to 100 feet away.
In the updated Technology Appraisal, people with ventricular arrhythmias are eligible for an ICD, which can help prevent cardiac arrest in those who have previously survived a life-threatening arrhythmia. In people with heart failure, CRT can improve life expectancy and quality of life.
The American Heart Association has announced its national officers for the 2014-15 fiscal year, which begins July 1.
The CRYSTAL AF trial found that continuous cardiac monitoring with the Reveal XT insertable cardiac monitor was superior to standard care at detecting atrial fibrillation (AF) in patients who have had strokes of undetermined causes.
Offering patients anticoagulants could help prevent thousands of strokes and premature deaths from atrial fibrillation (AF), according to NICE.
The ANSWER study evaluated whether the minimisation of Vp using the SafeR algorithm improves clinical outcomes for dual-chamber pacemaker patients implanted for sinus node disease or for atrio-ventricular block.
By Angela Gonzalez
The concept of a totally self-contained intracardiac pacemaker, first explored over 40 years ago by J W Spickler et al, has finally become a reality with the implantations of the Nanostim leadless pacemaker (St Jude Medical) and...
The need to perform defibrillation testing at the time of implantable cardioverter defibrillator (ICD) surgery has been the focus of significant discussion and debate over the past years. A number of retrospective studies, prospective series, and recently, the results of the long-awaited randomised SIMPLE trial have further suggested that this may be an unnecessary procedure, Jeanne E Poole writes for Cardiac Rhythm News.
At Cardiostim/EHRA Europace (18-21 June, Nice, France), Ilan Goldenberg presented an update on one-year follow-up results from the prospective WEARIT-II registry of 1,404 patients using the Lifevest wearable cardioverter defibrillator (Zoll) who were enrolled in the USA from August 2011 through December 2013.
Preliminary results from the first four human procedures with the Micra Transcatheter Pacing System (Medtronic) have shown the device has been successfully implanted with no major complications post-implant.
The RE-CIRCUIT study will investigate the safety and efficacy of uninterrupted anticoagulant treatment with Pradaxa in patients with atrial fibrillation who undergo ablation. Results of the study are expected in 2016.
Schwarzer Cardiotek has announced that it has completed the integration of the two companies. For the first time, the company showcased the combined portfolio of the merged entity and introduced its new corporate identity at Cardiostim/EHRA Europace (18-21 June, Nice, France).
New data from the Medtronic Adaptive CRT trial show a 61% (p=0.01) lower risk of atrial fibrillation (AF)-related problems in patients who receive a cardiac resynchronisation therapy-defibrillator (CRT-D) with the Medtronic-exclusive AdaptivCRT algorithm compared to conventional biventricular pacing therapy.
The award, which was chosen by a congress committee of electrophysiologists and conveys the support of the electrophysiology community, recognises the Nanostim leadless pacemaker as the industry's most innovative product in the sector of electrophysiology and cardiac techniques.
Updated National Institute for Health and Care Excellence (NICE) guidelines no longer recommend aspirin use alone, solely to reduce the risk of stroke in patients with atrial fibrillation (AF).
Men who eat moderate amounts of processed red meat may have an increased risk of incidence and death from heart failure, according to a study in Circulation: Heart Failure, an American Heart Association journal.
A new population-based study confirms previous study reports that radiofrequency catheter ablation is a safe method of arrhythmia treatment in children with long-term cumulative efficacy exceeding 90%, and a highly significant decrease in the procedure and fluoroscopy time during the study period.
Luis Aguinaga and Roberto Keegan give an overview on the design and initial results of the first Latin American catheter ablation of cardiac arrhythmias registry led by the Latin American Society of Electrophysiology and Cardiac Stimulation (SOLAECE).
Stereotaxis has announced that it has submitted a 510(k) Premarket Notification to the US Food and Drug Administration (FDA) for the company's Vdrive robotic navigation system with V-CAS catheter advancement system.
The Sensei X2 features faster image processing and a slimmer design with the stability and reachability of its predecessor. The improved processing and imaging capabilities of the Sensei X2 will also be available to users of the current Sensei X upon release.
The Stroke Feasibility Study is the first US clinical study to evaluate the safety of a novel epicardial-based left atrial appendage closure device for stroke prevention in atrial fibrillation patients.
New research in the USA finds that the societal burden of sudden cardiac death is high relative to other major causes of death.
eCardio Diagnostics and Shandong Yocaly Information Science & Technology, headquartered in Jinan, China, have recently signed a strategic cooperation agreement focused on furthering the advancement of remote cardiac monitoring products and services in the United States, China and beyond.
An examination of the benefit of preventive placement of implantable cardioverter-defibrillators (ICDs) in patients with a less severe level of heart failure finds significantly better survival at three years than that of similar patients with no ICD, according to a study in the June 4 issue of JAMA.
In her presentation at the German Cardiac Society's annual conference, Karin Nentwich (Bad Neustadt, Germany) concluded that implantable defibrillators are not contraindicated for young people engaging in physical education, sports and-within limits-competitive sports.
Medtronic has announced CE mark approval and commercial launch of the Advisa and Ensura SR MRI SureScan single-chamber pacemaker devices in Europe. Both pacemakers are approved for magnetic resonance imaging (MRI) scans positioned on any region of the body.
St Jude Medical has announced it has completed its acquisition of privately held CardioMEMS, developer of the CardioMEMS heart failure system. The acquisition was completed on 30 May 2014.
AliveCor has announced that in partnership with more than 100 pharmacies across North East London, free heart checks are being offered as part of Heart Rhythm Week, an international campaign from 2-6 June 2014. Participating pharmacies can screen for AF using the AliveCor heart monitor. Blood pressure screenings are also being offered to screen for hypertension.
The US Food and Drug Administration has approved the CardioMEMS heart failure system that measures the pulmonary artery pressures and heart rates of patients with New York Heart Association (NYHA) Class III heart failure who have been hospitalised for heart failure in the previous year. The device allows health care professionals to monitor the condition of their patients remotely.
John D Day, president-elect of the Heart Rhythm Society (HRS) and programme chair HRS Scientific Sessions 2014, talks to Cardiac Rhythm News about the highlights of this year’s congress including data from the SIMPLE trial, ARREST AF study and...
Biotronik has announced receiving CE mark approval for its ProMRI technology for ultra high field 3.0 tesla (T) and full-body magnetic resonance imaging (MRI) with the standard 1.5 T scan strength.
Spectranetics has announced receiving CE mark approval for its TightRail Rotating dilator sheath platform for mechanical cardiac lead extraction procedures.
iRhythm, has announced that it closed a US$17 million Series E financing led by Novo A/S. Norwest Venture Partners, which led the company's Series D financing, also participated in the oversubscribed round
Boehringer Ingelheim has announced the next step in the clinical development of idarucizumab, the investigational antidote for rapid reversal of dabigatran-induced anticoagulation
An 11-year study shows that hospitalisations for atrial fibrillation jumped by 23% and costs rose by 24%. The rise in atrial fibrillation's accompanying risk factors might account in part for the rise in hospitalisations.
Home telemonitoring is equally effective in ICD and CRT-D patients, a subanalysis of the IN-TIME trial has shown.
Results from the MINERVA (Minimize right ventricular pacing to prevent atrial fibrillation and heart failure) study have found that the Reactive ATP algorithm from Medtronic reduced the development of persistent atrial fibrillation by a 58% relative reduction compared to standard pacemakers (p<0.001).
Patients using the Boston Scientific Latitude remote patient management system with wireless telemetry demonstrated significantly lower mortality and fewer hospitalisations than patients with Latitude-compatible devices who were not followed on the system, according to results from the PREDICt-RM study (Patient related determinants of ICD remote monitoring utilisation and outcomes).
The expert consensus statement provides first-of-its-kind guidance on ICD therapy for the management of patient populations who are not well represented in clinical trials and, as a result, not specifically included in existing guidelines.
Medtronic has announced the results from the first prospective randomised clinical trial to show that Medtronic implantable cardioverter defibrillators (ICDs) can safely extend detection times before triggering therapy in secondary prevention patients.
A randomised controlled trial comparing three methods of pulmonary vein isolation for paroxysmal atrial fibrillation has revealed that procedures were faster and more effective with Medtronic's Arctic Front cryoballoon than wide encirclement using conventional point-by-point radiofrequency ablation. Procedures using the cryoballoon also resulted in a higher single procedure success rate.
The Entovis system allows patients to undergo Magnetic Resonance Imaging (MRI) scans with a limited exclusion zone. FDA approval covers both single chamber (SR-T) and dual chamber (DR-T) Entovis pacemakers when implanted with Setrox pacing leads.
Researchers in Australia have found that aggressive management of cardiovascular risk factors and weight can dramatically impact the long-term success rate of freedom from atrial fibrillation following ablation.
For stable clinical ventricular tachycardias, a substrate based ablation approach is more effective than conventional ablation preventing recurrences from any ventricular tachycardias in patients with ischaemic cardiomyopathy, according to results of the VISTA study, which is the first randomised trial performed in this area.
Results from the SIMPLE study show that despite the fact that defibrillation threshold testing at the time of device implantation is generally safe, it does not improve implantable cardioverter defibrillator (ICD) shock efficacy or reduce mortality compared to a no-testing strategy.
Data presented at the Heart Rhythm Society Annual Meeting support plans for phase 3 development of a fixed-dose combination of ranolazine and low-dose dronedarone in atrial fibrillation patients.
St Jude Medical announced that data presented during the 35th Heart Rhythm Society's Annual Scientific Sessions (7-10 May, San Francisco, USA), found that the use of quadripolar leads reduced the number of hospitalisations by 53% when compared to the non-quadripolar group. This hospitalisation rate reduction translated into a statistically significant 62% reduction in overall costs for both health care systems and patients.
Spectranetics has announced successful completion of the first live case using the TightRail rotating dilator sheath performed by Charles Love. The procedure, according to the company, represents a significant milestone as Spectranetics readies the TightRail tool for full launch later this month.
A first-of-its-kind study has found that bariatric surgery helps to prevent atrial fibrillation in patients with morbid obesity. The study was presented at the Heart Rhythm Society's 35th Annual Scientific Sessions (7-10 May, San Francisco, USA).
Richard Fogel, president-elect of the Heart Rhythm Society and chief executive officer at St Vincent Medical Group, considers he entered the field of electrophysiology at "the perfect time" when radiofrequency ablation and implantable cardiac defibrillators were emerging as a therapy to treat cardiac rhythm disorders. He spoke with Cardiac Rhythm News about this work, his interests in research and policy and the highlights of this year's Heart Rhythm Society's scientific sessions.
Results from the first prospective, randomised study of contact-force ablation technology for the treatment of paroxysmal atrial fibrillation met primary endpoints and supplement the growing body of evidence that supports the safety and effectiveness of contact-force ablation technology.
GLORIA-AF registry programme shows half of patients in China with an irregular heartbeat suboptimally protected against potentially devastating stroke.
A quarter century ago, doctors treating patients with implanted cardiac pacemakers had a big problem. Their patients were outliving the complex electrical devices that gave them an acceptable quality of life.
At the Heart Rhythm Society's 35th Annual Scientific Sessions, the Spectranetics Corporation will focus on management of cardiac device infections and debut of the company's first mechanical lead extraction devices.
CardioFocus has announced that recent data will be presented at two major conferences focusing on cardiac rhythm management, the 2014 Stanford Biodesign New Arrhythmia Technologies Retreat, and 2014 Heart Rhythm Society (HRS) Scientific Sessions.
Boston Scientific Corporation has joined Optum Labs as the Founding Medical Device Partner to help pioneer new research into effective treatments for heart failure and related cardiac conditions.
Clinical evidence about the company's cardiac rhythm management and ablation technologies will be provided in 38 sessions, including three late-breaking clinical trial sessions.
Eliquis (apixaban) has been recommended for continued use for prevention of stroke / systemic embolism in patients with non valvular atrial fibrillation while undergoing cardioversion by European Medicines Agency's Committee for Medicinal Products for Human Use.
In final draft guidance, the National Institute for Health and Care Excellence (NICE) has clearly defined which implantable cardiac devices are the most clinically and cost effective. The fresh guidance will help people with a variety of heart conditions including those with an irregular heartbeat.
Inspire therapy is a fully implanted neurostimulation device, the first of its kind for sleep apnoea, that provides an alternative treatment to continuous positive airway pressure that is proven, convenient and easy to use.
Medtronic has announced CE mark receipt and the European launch of its newest cardiac resynchronisation therapy-pacemaker, Viva CRT-P.
At the 10th annual congress of the European Cardiac Arrhythmia Society (ECAS; 23-25 March, Munich, Germany), Fu Siong Ng (London, UK) and colleagues won the first prize for best oral abstract with their research on the role of M-cells in electrophysiology. Ng writes for Cardiac Rhythm News an overview of the subject and the results of their own research.
Researchers studying the genetic aspects of cardiac arrhythmias have won second and third prizes for the best oral abstracts at the 10th Annual Congress of the European Cardiac Arrhythmia Society meeting in Munich, Germany.
John C Lincoln North Mountain Hospital in Phoenix, Arizona, USA, is the first hospital in Arizona, and only one of eight hospitals in the USA, utilising the MediGuide technology to reduce or eliminate radiation for minimally invasive cardiac procedures, according to a press release from John C Lincoln Health Network.
The financing, which was led by New Enterprise Associates and supported by existing investor, NBGI Ventures, will enable the company to continue to advance its next generation TempaSure ablation catheters and related clinical development programme.
A single centre analysis of antiarrhythmic drug therapy in older patients with atrial fibrillation and coronary artery disease has found it is associated with increased rehospitalisation at one year, and recommends safer and more effective therapies for symptom control in this group.
There are many risks and pitfalls with the current cardiac resynchronisation therapy devices which underscore the need for innovative approaches. Christopher V DeSimone and Samuel J Asirvatham, Mayo Clinic, Rochester, USA, write about a novel pacing lead (developed at Mayo Clinic by Asirvatham, Paul A Friedman, and Charles J Bruce) to help optimise pacing and synchronisation capabilities.
Foetal heart experts working with the American Heart Association have developed guidelines to help healthcare providers care for unborn babies with heart problems, as well as their families.
Physicians reporting 15 years of experience in extracting implantable cardioverter defibrillator (ICD) leads with a mechanical single-sheath technique and a multiple venous entry-site approach say they have found it is a complex but safe procedure, with a 99% success rate and no major complications.
Medtronic has announced the first US implant of the Evera MRI SureScan implantable cardioverter defibrillator (ICD) System, following US Food and Drug Administration (FDA) approval for its investigational device exemption application and pivotal clinical trial protocol.
Boston Scientific has announced that it has conducted the first implant in the NAVIGATE X4 clinical trial of the next generation Acuity X4 left-ventricular (LV) pacing leads and Reliance 4-Front defibrillation (ICD) leads.
CardioFocus has announced that leading cardiologists across Germany will gather to discuss clinical experiences with the HeartLight Endoscopic Ablation System for the treatment of atrial fibrillation on 23 April as part of the programme for the 80th Annual Meeting of the German Cardiac Society in Mannheim, Germany.
Boston Scientific has received FDA approval for its latest generation of defibrillators and heart failure devices. The newly approved devices include the Dynagen Mini and Inogen Mini ICDs, as well as the Dynagen X4 and Inogen X4 CRT-Ds.
Providence Saint Joseph Medical Center is the first hospital in the USA to conduct a magnetic resonance imaging (MRI) scan of a patient implanted with a new MRI-compatible pacemaker.
The US Food and Drug Administration (FDA) approved an application from Medtronic for revised labelling for two cardiac resynchronisation pacemakers (CRT-P) and eight cardiac resynchronisation defibrillators (CRT-D), expanding the indication for use to patients with atrioventricular (AV) block and less severe heart failure.
Spectranetics announced US Food and Drug Administration (FDA) clearance of two new mechanical lead extraction platforms - the TightRail rotating mechanical sheath and the SlightRail manual dilator sheath - that expand physicians' options for safe removal of cardiac leads.
Medtronic has announced CE mark approval and the European launch of the Evera magnetic resonance imaging (MRI) SureScan implantable cardioverter-defibrillator (ICD) system, the first and only ICD system approved for magnetic resonance imaging scans positioned on any region of the body.
The SEARCH-AF study conducted in Australia screened 1,000 patients over 65 years old for an abnormal heart rhythm, atrial fibrillation, in 10 community pharmacies in suburban Sydney, Australia. The AliveCor heart monitor was used to capture 30-60 second electrocardiogram (ECG) recordings and wirelessly transmit the recordings to study cardiologists.
Biotronik announced that the first patients across the United States have received implants of the new Iforia implantable cardioverter-defibrillators (ICDs).
Boston Scientific Corporation has expanded the launch of its S-ICD system into parts of Asia. The first implant of the S-ICD system in Asia was performed in Hong Kong by Hung Fat Tse, professor of cardiovascular medicine, The University of Hong Kong and Department of Medicine, Queen Mary Hospital in Pokfulam, Hong Kong, under the proctorship of Martin Stiles, director of Electrophysiology, Waikato Hospital in Hamilton, New Zealand.
The extension of the registry will include a specific focus on the use of novel oral anticoagulant therapy including prescribing patterns, providing new insight into the long-term management of patients with atrial fibrillation.
Poor sleep doubles hospitalisations in heart failure, according to new research in nearly 500 patients presented at EuroHeartCare (4-5 April, Stavanger, Norway).
A study published ahead-of-print in Circulation Arrhythmia and Electrophysiology has found that Brugada syndrome ECG is more prevalent among patients with schizophrenia than patients without schizophrenia. Use of sodium-channel blocking antipsychotics is not attributable to this prevalence.
Stereotaxis has announced that it has submitted a 510(k) Premarket Notification with the US Food and Drug Administration (FDA) for the company's Vdrive robotic navigation system with V-Loop variable loop catheter manipulator.
The first patient has been enrolled in the largest planned phase 3 study evaluating novel oral anticoagulant edoxaban in non-valvular atrial fibrillation patients undergoing electrical cardioversion.
The interim analysis of the ongoing EFFORTLESS S-ICD registry, which evaluated 456 patients with a mean follow-up of 558 days, is the first real-world study to date and confirms the clinical benefits of the S-ICD System in a broad range of patients at risk of sudden cardiac arrest.
In the longest follow-up to date of cardiac resynchronisation therapy (CRT) for mild heart failure patients, Boston Scientific's multicentre automatic defibrillator implantation trial - cardiac resynchronisation therapy (MADIT-CRT) study demonstrated significant and sustained survival benefit for the indicated population.
iRhythm Technologies announced new study findings that demonstrate the clinical utility of the ZIO Service for ruling in and ruling out cardiac arrhythmias in patients suspected of having the condition, following their discharge from the hospital emergency department.
The 2014 Guideline for the Management of Patients With Atrial Fibrillation includes recommendations for an increased use of radio frequency ablation in the treatment of non-valvular atrial fibrillation, the addition of three new anticoagulants to treatment options, a diminished role in the use of aspirin, and a more comprehensive risk calculator.
Biotronik announced CE mark approval for its new Eluna pacemaker series. The new generation of pacemakers includes single and dual-chamber as well as cardiac resynchronisation (CRT-P) devices. The first implantations are currently taking place worldwide.
Leadless pacing has shown to be safe and feasible with the Nanostim (St Jude Medical), self-contained, single-chamber leadless cardiac pacemaker, according to the LEADLESS trial results, recently published in Circulation.
New results from a sub-analysis of the TRUST trial, published in the European Heart Journal, show that remote monitoring of implantable cardioverter defibrillator (ICD) patients improves care and strengthens patient-physician links.
Subgroup analyses of East Asian populations from two phase 3 studies, ENGAGE AF-TIMI 48 and Hokusai-VTE, showed consistent results compared to the global study populations from these trials.
The US Food and Drug Administration (FDA) has approved the Allure Quadra cardiac resynchronisation therapy pacemaker (CRT-P), and the Assurity pacemaker and Endurity pacemaker families of devices.
St Jude Medical announced the global launch of the Optisure defibrillation lead. The Optisure lead features additional Optim insulation at the proximal end of the lead, including under the superior vena cava shock coil.
A subanalysis within the RAFT trial has shown significantly reduced hospitalisations with implantable cardioverter defibrillators with cardiac resynchronisation therapy (ICD-CRT) devices compared with ICD alone devices in heart failure patients.
Based on randomised trial data and from the UK payer perspective, apixaban is a cost-effective alternative to warfarin and aspirin for stroke prevention in atrial fibrillation patients.
The purpose of the large European clinical trial is to continue building evidence supporting the safety profile and performance of Nanostim.
Over-the-counter orders for the AliveCor Heart Monitor, the only US Food and Drug Administration (FDA) cleared mobile ECG recorder that supports both iPhone and Android smartphones, will now begin shipping.
Sorin Group has announced the purchase of Oscor Inc lead business, including a lead manufacturing facility in the Dominican Republic for an aggregate value of approximately US$20 million (€15.4 million).
The Ingevity family offers a comprehensive set of leads that can be placed using a 6 French introducer, including passive and active fixation models. Ingevity MRI pacing leads are part of the ImageReady MR-conditional pacemaker system.
The "I'm In" campaign will help to educate underrepresented populations about clinical trial participation in USA.
Ilan Goldenberg has coordinated investigator sponsored studies in the evaluation of cardiac rhythm devices in Israel. He chaired a session on investigator sponsored research at the 12th International Dead Sea Symposium (IDSS) on Innovations in Cardiac Arrhythmias and Device Therapy (Tel Aviv, Israel, 3-5 March) and spoke to Cardiac Rhythm News about the subject.
Biotronik has announced that the Food and Drug Administration (FDA) has approved the expansion of the company's ongoing ProMRI trial. The new phase of the trial (Phase C) will study Biotronik's ProMRI technology in implantable cardioverter defibrillator (ICD) devices.
A new study has found that new onset atrial fibrillation after transcatheter aortic valve implantation (TAVI) does not increase significantly the rate of stroke or mortality at 30-days and one-year follow-up.
Until now, knowledge on sudden cardiac death in children (1-18 years of age) has been sparse. Bo Gregers Winkel and Jacob Tfelt-Hansen write on the results of a retrospective study that looked at this subject in Denmark.
The GLORIA-AF registry is expected to provide meaningful knowledge on the global role and use of antithrombotic treatments in protecting patients with atrial fibrillation from the devastating effects of stroke.
Outbursts of anger may trigger heart attacks, strokes and other cardiovascular problems in the two hours immediately afterwards, according to the first study to systematically evaluate previous research into the link between the extreme emotion and all cardiovascular outcomes.
More than 100 countries have now approved Boehringer Ingelheim's Pradaxa for the prevention of stroke and systemic embolism for adult patients with non-valvular atrial fibrillation.
Biotronik has announced CE mark approval and the first implantations of the Sentus ProMRI lead. Biotronik's bipolar cardiac resynchronisation therapy lead is the first MR conditional lead with a 4 french diameter-approximately 1.6 millimetres.
Richard Bernstein, professor of neurology in the Davee Department of Neurology at Northwestern University, director of the Stroke Program at Northwestern Memorial Hospital in Chicago, USA, and primary investigator of the CRYSTAL AF study, speaks at the International Stroke Conference...
Under the agreement, Hokushin Medical will market, sell and distribute the Niobe Magnetic Navigation system and disposable devices to Japanese customers, as well as provide customer training and clinical support.
The first catheter ablation therapy in the US to feature direct contact force technology for treatment of atrial fibrillation, has been approved by the US Food and Drug Administration.
The subanalysis shows that apixaban was more effective than warfarin in reducing the risk of stroke or systemic embolism, was associated with less major bleeding, less total bleeding and less intracranial haemorrhage, regardless of age and in reducing all-cause mortality across age groups.
Scripps Green Hospital has become the first hospital in the United States to implant the Reveal Linq insertable cardiac monitor.
The partnership between AliveCor and Practice Fusion enables customers of free, web-based electronic health records to seamlessly import AliveCor System's electrocardiogram readings.
Medtronic announced the first US implant of the Micra transcatheter pacing system, which is one-tenth the size of a conventional pacemaker, and comparable in size to a large vitamin.
Results from the CRYSTAL AF trial have shown that atrial fibrillation in patients with cryptogenic stroke is better detected with insertable cardiac monitors than standard monitoring at six, 12 and 36 months.
Nick Linker, Middlesbrough, UK, recently implanted, for the first time in the UK, the smallest version of an insertable loop recorder. He spoke to Cardiac Rhythm News on the role and advantages of implantable loop recorders in the diagnosis of syncope and cardiac arrhythmias, the main features of this new device and its cost-effectiveness.
Medtronic has announced US Food and Drug Administration (FDA) 510(k) clearance, CE mark, and the global launch of its Reveal Linq insertable cardiac monitor system, a small, wireless monitor that provides long-term remote monitoring to help physicians diagnose and monitor irregular heartbeats.
Apama Medical announced receipt of a Chinese notice of allowance for claims covering their low profile electrode assembly that enables the attachment of flexible electrodes to an ablation balloon for energy delivery and monitoring.
Even though novel oral anticoagulants present an "amazing opportunity to improve stroke prevention in atrial fibrillation", according to Peter Kowey (Philadelphia, USA), there are still several limitations with regards to monitoring, discontinuation, lack of reversal agents and lack of long-term data that affect their widespread use.
Stereotaxis announced that its board of directors has appointed Chairman William C Mills III to chief executive officer, effective February 12, 2014. Mills has been serving as interim chief executive officer since April 2013.
A poster presented at the 43rd Annual Meeting of the German Society for Thoracic and Cardiovascular Surgery has shown that vernakalant (Brinavess, Cardiome) successfully cardioverted 74% of atrial fibrillation patients to sinus rhythm at 90 minutes.
AtriCure has announced the completion of the previously publicised acquisition of Endoscopic Technologies. Estech develops and markets temperature controlled radiofrequency energy technologies for the treatment of atrial fibrillation.
The Heart Rhythm Society developed the list as part of Choosing Wisely, an initiative of the American Board of Internal Medicine Foundation launched in 2012 aimed at curbing the use of certain tests and procedures that are not supported by clinical research.
New study data support the use of the Zio Patch to help identify underlying cardiac arrhythmias in patients who have had a stroke or transient ischaemic attack. The Zio Patch enables long-term continuous monitoring to detect sporadic heart rhythm disturbances, including atrial fibrillation.
The BERLIN study is a prospective, multicentre study that seeks to determine the best time for catheter ablation in the treatment of patients with sustained ventricular tachycardia and coronary artery disease.
AliveCor has announced that the US Food and Drug Administration (FDA) has granted the company over-the-counter clearance for its AliveCor Heart Monitor, a single-channel ECG recorder, previously available by prescription only.
Cardiac Rhythm News spoke to Jeanne Poole, one of the founding members of the steering committee of the EPIC Alliance, about the creation, objectives and key achievements of this organisation that empowers women working or wanting to work in electrophysiology.
Heart failure patients treated in the WISE-CRT study with a leadless ultrasound-based device demonstrated a significant improvement of functional status and a significant increase of left ventricular ejection fraction at six-month follow-up.
St Jude Medical has announced the first US implant in the company's LEADLESS II pivotal trial designed to evaluate the Nanostim leadless pacemaker for US Food and Drug Administration (FDA) approval.
According to a recent study, aspirin is still overprescribed for stroke prevention in atrial fibrillation despite the potential for dangerous side effects.
Rome will be the host of the European Society of Cardiology Congress 2016.
The diagnostic testing facilities provide 24-hour monitoring services for remote cardiac monitoring prescribed by physicians, including mobile cardiac telemetry, cardiac event monitors and holters.
Israel Ministry of Health has approved clinical trials of the CardioFit system in patients with chronic heart failure.
Patients implanted with the Advisa DR MRI or Revo MRI SureScan pacing systems now can have MRI scans without positioning restrictions, including the chest area, which previously had been restricted.
St Jude Medical has announced the first post-approval implant of its Nanostim leadless pacemaker in the UK. Richard Schilling implanted the pacemaker at St Bartholomew's Hospital in London, UK.
Biotronik has announced that that the first patients have been enrolled in an expansion of its ongoing ProMRI trial. The aim of the study is to evaluate the safety and effectiveness of the company's pacing systems in patients undergoing full-body MRI.
Hans Kottkamp, Zurich, Switzerland, presented a new device strategy that combines diagnostic and treatment capabilities for atrial fibrillation in one system, at the AF Symposium in Orlando USA (9-11 January 2014). He spoke to Cardiac Rhythm News about the combined system.
Douglas Packer (Mayo Clinic, Rochester, USA) shares with Cardiac Rhythm News information on the design and rationale of the CABANA (Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation) trial, at the AF Symposium in Orlando, USA (8-11 January 2014).
According...
The draft guidance from the UK's National Institute for Health and Care Excellence (NICE) defines which implantable cardiac devices are clinically and cost effective options for people with life-threatening arrhythmias or heart failure.
Mark Earley (London, UK) argues that despite the breathtaking progress of interventional electrophysiology in the last 10 years, ablation of persistent atrial fibrillation remains an unconquered summit.
The FDA has approved St Jude Medical's Therapy Cool Flex Ablation Catheter and Cardiac Ablation Generator for the treatment of atrial flutter.
Anne Curtis is past president (2005-2006) of the Heart Rhythm Society. She has been involved in various clinical trials in electrophysiology including her most recent trial, BLOCK HF, and is currently a member of different editorial boards in the field. She spoke to Cardiac Rhythm News about her career achievements, memorable cases and the major challenges executing a trial.
New data from the ECOST study show remote monitoring with Biotronik Home Monitoring significantly reduces the cost of follow-up in patients with implantable defibrillators (ICDs).
CardioFocus has announced that its HeartLight endoscopic ablation system is now available in Spain and Hospital Universitari i Politecnic La Fe in Valencia was the first in the country to use the technology.
Biotronik has announced that its devices and leads, including the latest DX system technology, has been added to the list of cardiac rhythm management device suppliers to the US Department of Veterans Affairs.
Tyrx's product offerings include the recently FDA cleared Aigisrx R Fully Resorbable Antibacterial Envelope, designed to reduce surgical site infections associated with cardiac implantable electronic devices, and the Aigisrx N Antibacterial Envelope, for use with spinal cord neuromostimulators.
Results of the 120-patient study, which was conducted at three sites in the USA and two in Europe, will be included in a 510(k) premarket notification the company intends to submit in the first quarter of 2014.
The new system processes information in seconds, providing near-instantaneous intra-procedural mapping and re-mapping capabilities. In addition, the system incorporates a new color-imaging module to aid identification of "Rotors," an electrophysiologic phenomenon previously shown to sustain atrial fibrillation.
The study will evaluate the safety of the Phased Radiofrequency system (Medtronic) for the treatment o patients with persistent or long-standing atrial fibrillation.
Researchers in Germany have reported that depressed mood tends to be more severe in patients with persistent atrial fibrillation than in patients with paroxysmal atrial fibrillation. The study comparing populations from two large, recent, controlled clinical trials in atrial fibrillation was published ahead-of-print in Europace.
On 2 January, AtriCure announced the completion of the previously announced acquisition of Endoscopic Technologies (Estech), a developer of surgical ablation devices with controlled radiofrequency technology used in the treatment of atrial fibrillation.
Despite new algorithms for better discrimination of supraventricular from ventricular tachyarrhythmias being constantly developed, Helmut U Klein writes that the problem of inappropriate implantable cardioverter defibrillator (ICD) therapy remains unsolved.
Updated results of the EFFORTLESS S-ICD registry have shown the Subcutaneous Implantable Cardioverter Defibrillator (S-ICD, Boston Scientific) continues to show effective conversion of induced and ambulatory ventricular tachycardia or fibrillation (VT/VF) comparable to standard ICD performance and low complication rates.
AtriCure has announced entering into a definitive merger agreement to acquire Estech, a developer of surgical ablation devices with controlled radiofrequency technology used in the treatment of atrial fibrillation.
Daiichi Sankyo is seeking approval in Japan for edoxaban in new indications for non-valvular atrial fibrillation and symptomatic venous thromboembolism.
A new classification of atrial fibrillation (AF) by electrocardiogram (ECG) is set to be developed by European experts to aid personalised management of condition.
Results of EORP-AF, a pilot registry on the management and treatment of atrial fibrillation in Europe, have shown that compliance with treatment guidelines for atrial fibrillation patients with the lowest and higher stroke risk scores remains suboptimal.
The UK charity Heart Research UK has awarded a grant of £125,000 to the National Heart and Lung Institute at Imperial College, London, UK, for research of new drug treatments to target cells that cause cardiac arrhythmias.
Andreas Goette (Paderborn, Germany) explains the results of a study which found how rivaroxaban (Xarelto, Bayer Healthcare) helps to prevent prothrombogenic remodelling of human atrial myocardium.
A first-of-its-kind meta-analysis comparing four new oral anticoagulants with warfarin has found they perform better than warfarin reducing stroke, intracranial haemorrhage and mortality in atrial fibrillation patients. The study was published ahead-of-print in The Lancet.
Boston Scientific has announced it has received CE mark approval of its X4 line of quadripolar CRT-D systems including Autogen X4, Dynagen X4, and Inogen X4 cardiac resynchronisation therapy defibrillators (CRT-Ds), a suite of Acuity X4 quadripolar left ventricular (LV) leads and the Acuity Pro lead delivery system.
On 11 December, the FDA's Circulatory System Devices Panel of the Medical Devices Advisory Committee voted favourably by a majority, Yes: 13, No: 1, that the benefits of the Watchman left atrial appendage closure device outweigh the risks.
Preliminary results of a new multicentre study have found that both paroxysmal and persistent atrial fibrillation may be sustained by localised stable rotors and that targeted ablation of those sources by focal impulse and rotor mapping (FIRM) can eliminate atrial fibrillation
On 9 December, Medtronic announced the first-in-human implant of its Micra Transcatheter Pacing System (TPS). According to the company, this is the world's smallest pacemaker and does not require transvenous leads.
Researchers in the USA and Hong Kong have developed a two-step system that helps to differentiate human embryonic stem cells from induced pluripotent stem cells. This process results in high efficiency and reproducibility of heart muscle, which mimics normal cardiovascular development.
Eugenio Picano, CNR, Institute of Clinical Physiology, Pisa, Italy, writes for Cardiac Rhythm News a summary of the paper: "The appropriate and justified use of medical radiation in cardiovascular imaging. A position document of the ESC associations of cardiovascular imaging, percutaneous cardiovascular interventions and electrophysiology", recently published in the European Heart Journal. He also spoke on the subject at Venice Arrhythmias (27-29 October, Venice, Italy).
Certain HeartStart automated external defibrillator (AED) devices made by Philips Healthcare may be unable to deliver needed defibrillator shock in a cardiac emergency situation, the US Food and Drug Administration (FDA) has announced.
The global cardiac rhythm management (CRM) market is set to experience significant expansion and emerging market adoption, with revenues climbing from US$10.9 billion in 2012 to more than US$15.7 billion by 2020.
Sorin has announced the CE mark approval and first implant of the Kora 100 pacing system. The new device enables implanted patients to undergo magnetic resonance imaging (MRI) safely.
Luigi Di Biase writes for Cardiac Rhythm News on the results of a study that identified four different left atrial appendage morphologies and its relationship with the risk of stroke in atrial fibrillation patients. "These results could have a relevant impact on the oral anticoagulation management and occlusion device management of atrial fibrillation patients with an intermediate risk for stroke," writes Di Biase.
The study will evaluate the use of the AtriClip left atrial appendage exclusion system to prevent stroke in patients with atrial fibrillation as alternative to anti-coagulation therapy.
AliveCor has launched a mobile heart health service, offering expert review of electrocardiograms recorded through their smartphone app. Patients can share these expert reviews and their electrocardiograms with their physicians. Physicians in turn can manage their patients through AliveCor.com, using their PC or tablet.
On 4 November the 1,000th patient was enrolled in the AFNET-EAST trial. The pan-European clinical trial compares two different treatment strategies in atrial fibrillation EAST trial compares early rhythm control therapy to usual care.
New research shows that pacemakers with enhanced-pacing features (Medtronic) have the ability to slow the progression of atrial fibrillation in patients with bradycardia, or a slow heartbeat.
ChanRx has announced positive safety and statistically significant efficacy data from a phase IIb study of vanoxerine (GBR-12909), a drug in development for the treatment of atrial fibrillation. The data were presented yesterday at the American Heart Association 2013 Scientific Sessions (16-20 November, Dallas, USA).
Results from the ENGAGE AF-TIMI 48 clinical trial have found that the investigational, oral, once daily direct factor Xa-inhibitor edoxaban (Lixiana, Daiichi-Sankyo) met the primary efficacy endpoint of non-inferiority compared to warfarin for the prevention of stroke or systemic embolic events in patients with non-valvular atrial fibrillation.
A study presented at the American Heart Association Scientific Sessions (16-20 November, Dallas, USA) has shown that mechanical chest compressions in combination with defibrillation during ongoing compressions provided no improved four-hour survival vs. manual cardiopulmonary resuscitation in patients with out-of-hospital cardiac arrest.
A new study published in the Journal of the American Medical Association has shown that a structured weight reduction programme for highly symptomatic atrial fibrillation patients helps to reduce symptom burden and severity and also helps to reduce antiarrhythmic drug use.
CardioInsight has announced that it has completed a long-term strategic financing that provides the company with an initial investment of US$15 million.
The resting pulse rate of UK pre-teens may have risen by up to two beats a minute during the past 30 years. But the rise does not seem to be linked to the overall weight gain seen in this age group during this period, reveals research published online in the Archives of Disease in Childhood.
A first-of-its-kind study has found that foetal magnetocardiography (fMCG)-the magnetic analogue of electrocardiograms (ECG)-may help to diagnose and possibly treat in utero long QT syndrome.
Boehringer Ingelheim has announced the upcoming presentation of the latest efficacy and safety data for the novel oral anticoagulant dabigatran etexilate (Pradaxa) at the 2013 American Heart Association's (AHA) Scientific Sessions (16-20 November, Dallas, USA).
Martek Medical has released the Lifeline Pro automated external defibrillator (AED) in the UK market. The Lifeline Pro AED (Defibtech), according to a company release, has the quickest charge to shock time of any defibrillator on the market at just four seconds.
On 7 November, Topera announced receiving 510(k) clearance by the FDA for the Firmap catheter and the RhythmView 3D mapping workstation. This technology has been developed for the identification of the sources that sustain complex cardiac arrhythmias.
Radiofrequency ablation of supraventricular tachycardia guided by the non-fluoroscopic Ensite NavX mapping system (St Jude Medical) has shown lower levels of ionising radiation exposure for the patient and for the operator compared with conventional catheter ablation, according to preliminary results from the NO-PARTY Italian trial.
On 1 November, Boston Scientific closed on its previously announced agreement to acquire Bard EP, the electrophysiology (EP) business of CR Bard.
A new study published in the British Journal of Pharmacology has shown that a new medication based on resveratrol-a compound found in red wine and nuts-is an effective inhibitor of several potential targets involved in atrial fibrillation development. Cardiac Rhythm News spoke to Peter Light, lead author of the study.
EWOLUTION is a multinational, prospective, multicentre, post market registry aimed to collect data on procedural success, incidence of stroke and mortality of patients implanted with a Watchman device.
Through its CardioSmart patient initiative, the American College of Cardiology is hosting a contest to find six individuals who are living well with heart disease and can inspire others to take charge of their heart health.
The CHART-1 trial, according to a company release, represents the world's first phase III trial in regenerative medicine for a pre-programmed cellular therapy targeting heart failure.
Stereotaxis has announced that the Ministry of Health, Labor and Welfare (MHLW) in Japan has classified its Niobe Magnetic Navigation System as a C2 medical device.
The North American Thrombosis Forum (NATF) has launched an innovative educational initiative on risk assessment and anticoagulation for stroke prevention in atrial fibrillation.
Cardiome announced on 7 October the publication of positive data from an observational, retrospective study investigating Brinavess for atrial fibrillation. The study was performed at Skane University Hospital in Malmö, Sweden.
With the advent of new oral anticoagulants, there are many questions towards selection of the most appropriate treatment for patients with atrial fibrillation. Sean D Pokorney (Durham, USA) writes for Cardiac Rhythm News about the different clinical strategies to take into consideration based on the results of landmark clinical trials of novel oral anticoagulants.
An independent study demonstrates significant differences in battery longevity between cardiac resynchronisation therapy (CRT) implantable cardioverter defibrillators (ICDs), which are still implanted today worldwide.
Antonio Raviele (Venice, Italy), cofounder of the VeniceArrhythmias congress, implanted the first intravenous defibrillator lead in Italy. He spoke to Cardiac Rhythm News about this experience, his current research, his projects dedicated to enhance awareness on cardiac arrhythmias and the highlights of VeniceArrhythmias 2013
The randomised, controlled trial of the HeartLight Endoscopic Ablation System (Cardiofocus) completed enrolment of over 400 patients from 21 research sites across the United States.
On 14 October, St Jude Medical announced the completion of its acquisition of Nanostim, a privately-owned developer of miniaturised, leadless pacemakers.
The results of the EchoCRT study show that cardiac resynchronisation therapy (CRT) does not reduce the rate of death or hospitalisation and may increase mortality in heart failure patients with a QRS duration of less than 130msec
According to a company release, the Idova 7 technology offers ultrahigh-energy therapy without compromising on short charge times (10sec), smaller size (34cc), and longevity of more than 11 years.
On 8 October, the FDA's Circulatory Systems Devices Advisory Panel voted that biventricular pacing with Medtronic devices is beneficial for treating patients who have atrioventricular block and left ventricular systolic dysfunction, compared to conventional right ventricular pacing.
The guideline-recommended use of oral anticoagulation has increased compared to previous registries, as well as the use of antiarrhythmic drugs and catheter ablation procedures. Data have recently been published in Europace
Public health expenditure in Latin America is directed mainly to tackle diseases and social problems that kill the young population but not to treat cardiac diseases (including arrhythmias), which mainly affect older people, writes Diego Vanegas, Bogota, Colombia
Darryl Wells of the Swedish Heart and Vascular Institute, Seattle, USA, talks about how safe the ablation procedure is and how important getting patients home within 24 hours of the procedure is. "Our goal is to get patients off antiarrhythmic...
On 1 October, Biotronik announced the Food and Drug Administration (FDA) approval for its Ilesto family of implantable cardioverter-defibrillator/cardiac resynchronisation therapy defibrillators (ICD/CRT-D).
On 1 October, AtriCure launched its Maze IV training programme on surgical ablation for the treatment of atrial fibrillation at the Sana Stuttgart Cardiac Surgery Center in Germany.
On 23 September, Medtronic announced clinical trial results showing that heart failure patients treated with its AdaptivCRT feature experienced a 46% reduction in atrial fibrillation risk.
Patients with central sleep apnoea, treated with the remede system (Respicardia), experienced a 50% decrease in apnoea-hypopnea index, improved oxygenation by over 50%, decreased arousals and improved quality of life, according to results of the remede system pilot study presented by William T Abraham at the 17th Heart Failure Society of America meeting.
Patients treated with vernakalant achieved conversion to normal sinus rhythm in a median time of 12 minutes compared to 151 minutes for the propafenone group and 162 minutes for the flecainide group (p<0.01), according to results published in the Journal of Atrial Fibrillation.
The company announced that Thorsten Lewalter, director of the Isar Heart Center Munich, Medical Center for Cardiology and Internal Intensive Care, Munich, Germany, successfully performed the first AlCath Flutter case worldwide as part of an international product evaluation.
A new study has identified that, in anticoagulated patients with atrial fibrillation (AF), the HAS-BLED score has better prediction accuracy for major bleeding than the CHADS2 or CHA2DS2-VASc scores.
Seizure in patients with epilepsy may not be a trigger of sudden cardiac arrest (SCA). Eric Stecker, Knight Cardiovascular Institute, Oregon Health and Science University, Portland, USA, and colleagues, from the Cedars-Sinai Heart Institute, Los Angeles, USA, reported in a study recently published in Circulation Arrhythmia and Electrophysiology.
Christophe Leclercq, Rennes, France, chairperson of two European Society of Cardiology (ESC) 2013 sessions in cardiac resynchronisation therapy, speaks about this year’s congress highlights on arrhythmia and device therapy and also what is expected from the ESC congress 2014...
The Institute for Advancing Science will offer training in clinical practice and multidisciplinary programmes in Interventional Cardiology, Cardiac Rhythm Management and Electrophysiology, Endoscopy, Peripheral Interventions and Urology and Women's Health.
Screening athletes for the risk of sudden cardiac arrest has been a topic of discussion for many years. Jacob Tfelt-Hansen, author of a Danish study on this subject, gave an overview about different positions and studies that favour or disfavour this practice.
According to research by the Millennium Research Group, the Chinese electrophysiology market will grow approximately 20% in 2013, based on the largest available sampling of ablation procedures.
Atrial fibrosis detected using delayed enhancement magnetic resonance imaging (DE-MRI) seems to be an independent predictor of procedural outcome in patients undergoing ablation of atrial fibrillation, according to results of the DECAAF (Delayed enhancement-MRI determinant of successful catheter ablation of atrial fibrillation) study
SERVE-HF is an international, randomised study of 1,325 participants investigating if the treatment of central sleep apnoea improves survival and outcomes of patients with stable heart failure.
AtriCure has announced that Scott Drake, chief executive officer of Spectranetics has been elected as director. Donald C Harrison, one of the founders of AtriCure, has also informed the board of directors of his plans to retire at the 2013 Annual Meeting in May 2014.
Claudio Tondo (Milan, Italy) analyses the best treatment options for different kinds of idiopathic ventricular tachycardias. He will be talking about this subject at Venice Arrhythmias (27-29 October, Venice Italy).
A first of its kind study has shown that patients with atrial fibrillation who undergo catheter ablation have a lower stroke risk than patients who do not undergo the procedure independent of CHADS2 score.
A new technology that uses a sensor-based electromagnetic tracking system may help to guide cardiac resynchronisation therapy (CRT) device implantation safely and potentially cut radiation exposure time as compared with traditional fluoroscopy.
Survival for out-of-hospital cardiac arrest is just 7%, according to research presented at ESC congress 2013 by Xavier Jouven, Hopital Georges Pompidou, Paris, France and Wulfran Bougouin, Paris, France.
The WaveCrest is an implantable device that seals off the left atrial appendage (LAA) opening so clots cannot escape into the blood stream and cause a stroke.
The findings, from eight abstracts presented this week at the ESC congress 2013, collectively show that anticoagulant therapy which is known to significantly lower stroke risk in AF patients-is consistently under-utilised among those at-risk atrial fibrillation patients.
Results of a post-hoc subanalysis from the phase III ARISTOTLE trial have shown that stroke or systemic embolism and major bleeding were uncommon within 30 days in non-valvular atrial fibrillation patients who undertook a medical procedure.
Results from the IN-TIME study have shown a significant reduction in all-cause mortality in heart failure patients with implantable cardioverter defibrillators (ICDs) or cardiac resynchonisation therapy defibrillators (CRT-Ds) fitted with remote monitoring compared to standard care therapy.
The study, according to a company release, demonstrates that implantable cardioverter defibrillator (ICD) patients with Biotronik's Home Monitoring can enjoy a reduction in follow-up visits by 58%. This in turn leads to patients reporting enhanced quality of life.
A new trial has found that the subcutaneous implantable cardioverter defibrillator (S-ICD System, Cameron Health/Boston Scientific) is safe and effective at detecting and treating both induced and spontaneous ventricular tachycardia and ventricular fibrillation in a broad range of patients requiring implantable cardioverter defibrillators (ICDs).
The Catharina Hospital (Eindhoven, The Netherlands) and Royal Philips has announced the results of a clinical study involving the treatment of 136 patients with complex heart rhythm disorders such as atrial fibrillation.
Personalised management is the only way to close the mortality gap for patients with atrial fibrillation, according to an ESC consensus paper presented at ESC Congress 2013 by Paulus Kirchhof (UK).
The first patient implants of the Cognis (Boston Scientific) cardiac resynchronisation therapy defibrillator (CRT-D) were successfully performed in China's Zhejiang province and Beijing by Farong Shen in Zhejiang Lucheng Hospital and Wei Hua in Beijing Fuwai Hospital.
AtriCure and Century Medical of Japan have announced the extension and expansion of their multi-year agreement in which Century Medical will distribute AtriCure's surgical ablation technologies to hospitals in Japan.
Chern-En Chiang, General Clinical Research Center and Division of Cardiology, Taipei Veterans General Hospital, National Yang-Ming University, Taipei, Taiwan, and colleagues, investigated the appropriate uses of antiarrhythmic drugs in atrial fibrillation patients and reported that there were inconsistencies in drug prescription compared to guidelines issued in 2006.
Boston Scientific Corporation has announced it has received US Food and Drug Administration (FDA) approval of its IntellaTip MiFi XP catheter and 510(k) clearance of its Zurpaz 8.5F steerable sheath. The IntellaTip is indicated for ablation of atrial flutter.
A study undertaken by a team at the Massachusetts General Hospital, Boston, USA, suggests there is a need to individualise physicians' pacing approach for patients receiving cardiac resynchronisation therapy. Jagmeet Singh writes about the outcomes of this study.
St Jude Medical has announced the acquisition of Endosense. The acquisition adds to the company's electrophysiology portfolio. St Jude Medical has made an initial payment of approximately 159 million Swiss Francs (US$170 million) and acquired 100% of the outstanding equity of Endosense.
World-renowned cardiologist and researcher Valentin Fuster has been named editor in chief of the Journal of the American College of Cardiology, the flagship journal of the American College of Cardiology.
BioVentrix has announced the first use of its Revivent Myocardial Anchoring via less invasive ventricular enhancement (the LIVE) procedure in Germany. The successful procedure was performed on a 54-year old man suffering from advanced heart failure at the Schön Klinik Vogtareuth in Vogtareuth, Germany.
St Jude Medical announced on 15 August that the US Food and Drug Administration (FDA) has approved its MediGuide enabled ablation catheters. The catheter can be tracked using 3D visualisation on previously recorded fluoroscopic images in real time.
TYRX has announced that the first implantation of its new AIGISRx R fully resorbable antibacterial envelope has taken place at the Vanderbilt Heart and Vascular Institute in Nashville, USA, by Christopher R Ellis. The AIGISRx R antibacterial envelope received US Food and Drug Administration (FDA) clearance on 20 May 2013.
A new study published online in the Journal of the American College of Cardiology has found that people experiencing sudden cardiac arrest at exercise facilities have a higher chance of survival than at other indoor locations, likely due to early cardio-pulmonary resuscitation and access to an automated external defibrillator (AED), among other factors
Primary results from the PainFree SST study have shown that 97.6% of patients were free from inappropriate shocks with single-chamber implantable cardioverter defibrillators (ICDs) at one year of implantation.
ARTISAN-AF study is a pivotal clinical trial evaluating the use of Hansen Medical's Artisan family of control catheters with its Sensei X Robotic Catheter System for treatment of atrial fibrillation.
John D Day (Murray, USA) has performed more than 3,000 catheter ablation for atrial fibrillation procedures. Based on his experience, he describes five basic principles to minimise the risk of serious complications.
Patrick Ellinor, director Cardiac Step Down Unit, Massachusetts General Hospital Corrigan Minehan Heart Centre (Boston, USA) discusses different treatment options to prevent stroke in patients with atrial fibrillation.
https://youtu.be/Y1s_nCG2eYU
Healthcare professionals are urged to counsel heart and stroke patients on how to resume a healthy sex life, according to a joint statement published in the American Heart Association journal Circulation and the European Heart Journal.
On 1 August, eCardio Diagnostics announced the promotion of John H Untereker to president, effective immediately.
Trevena has announced the publication of new findings related to the mechanism of action of its Angiotensin II Type 1 Receptor (AT1R) biased ligands. The publication describes work led by R John Solaro, head of the Department of Physiology and Biophysics at the University of Illinois, Chicago, USA, performed in collaboration with Trevena scientists.
The Vdrive with V-Sono system (Stereotaxis) is indicated for the remote control of compatible intracardiac echocardiography or ultrasound catheters inserted into the right atrium for cardiac ablation procedures.
Physicians in the USA are using, for the first time, coil embolisation to treat severe ventricular tachycardias. The intervention is normally employed for treating brain aneurysms. Cardiac Rhythm News spoke to Kalyanam Shivkumar, co-author of the report in more detail about the technique, its benefits and most suitable patients for this procedure.
A substudy from the ANTIPAF trial has found that quality of life was overrated or underrated by physicians in 55% of patients with paroxysmal atrial fibrillation. Depression was the major contributor to a higher likelihood of overrating.
On 25 July, SynCardia Systems announced that 100 SynCardia temporary Total Artificial Hearts have been implanted by SynCardia certified centres in 2013.
The Rhythmia Mapping System (Boston Scientific) is a next-generation 3D mapping and navigation solution for use in cardiac catheter ablations and other electrophysiology procedures to diagnose or treat cardiac arrhythmias.
Jagmeet Singh, director of the Cardiac Resynchronization Therapy Program at the Massachusetts General Hospital Heart Center, USA, discusses a clinical trial which compared multidisciplinary care vs. conventional care for cardiac resynchronisation therapy patients.
https://youtu.be/PFRm9QwrS7g
Werner Jung (Villingen-Schwenningen, Germany) revises patients' opinions regarding remote monitoring of implantable cardiac defibrillators (ICDs). He spoke on the subject at the European Heart Rhythm Association (EHRA) Europace meeting (23-26 June, Athens, Greece).
On 17 July, Zoll Medical announced that it has been selected as a nominated supplier by the British Heart Foundation (BHF) for the Zoll AED Plus through 2016.
The consensus on paediatric arrhythmias is the first European statement concerning the diagnosis and management of paediatric arrhythmias. It is also the first joint document between European Heart Rhythm Association (EHRA) of the European Society of Cardiology (ESC) and the Association for European Paediatric and Congenital Cardiology AEPC.
Results from a new sub-analysis of the RE-LY trial support consistent benefit of dabigatran (Pradaxa, Boehringer Ingelheim) over warfarin in difficult-to-treat patients with non-valvular atrial fibrillation and previous symptomatic heart failure.
Juniper Research has announced that its latest report on the mHealth (mobile health) market forecasts cumulative cost savings from remote patient monitoring of up to US$36 billion globally over the next five years.
AtriCure has announced that David Francischelli has been appointed as vice president of Research and Development, effective 6 August 2013.
Despite the widespread nature of atrial fibrillation, our understanding of the disease remains limited according to Patrick T Ellinor, director Cardiac Step Down Unit, Massachusetts General Hospital Corrigan Minehan Heart Centre (Boston, USA) and associate professor, Harvard Medical School. He spoke to Cardiac Rhythm News about current research on genetics of atrial fibrillation.
There have been a number of studies conducted to assess the efficacy and adverse effects of radiofrequency ablation of atrial fibrillation, but very little data is available on the effect of radiofrequency ablation on the upper gastrointestinal system. Dhanunjaya Lakkireddy, Kansas City, USA, reviews this subject based on the results of a new systematic approach the AF GUT study.
AIGISRx R is a fully bioresorbable, antibacterial mesh envelope, intended to hold cardiovascular implantable electronic devices securely in place in order to provide a stable environment when implanted in the body.
A good night's sleep can increase the benefit of exercise, healthy diet, moderate alcohol consumption and non-smoking in their protection against cardiovascular disease, according to results of a large population follow-up study, the MORGEN study, published on 3 July in the European Journal of Preventive Cardiology.
According to a Mass General press release, this is the first hospital in New England -and the second in the USA-to pair renal artery sympathetic denervation with pulmonary vein isolation for patients with atrial fibrillation and hypertension.
Complete linear lesions when using catether ablation to isolate pulmonary veins have been shown to be more effective than incomplete lesions in preventing recurrence of atrial fibrillation, according to results from the GAP-AF study presented at a late-breaking trial session of the European Heart Rhythm Association (EHRA) Europace meeting (23-26 June, Athens, Greece).
According to results from the SARA study, presented at a late-breaking trial session of the European Heart Rhythm Association (EHRA) Europace meeting (23-26 June, Athens, Greece), catheter ablation therapy has been shown to be superior to medical therapy for maintenance of sinus rhythm in patients with persistent atrial fibrillation.
Boston Scientific has announced that it has entered into a definitive agreement to acquire Bard EP, the electrophysiology business of CR Bard, for US$275 million in cash. The company expects to close the transaction in the second half of 2013, subject to customary closing conditions.
The Accent MRI Pacing System was said, in a company release, allows the use of full-body scans using high-power imaging technology in patients with the pacemaker.
Outcomes of an analysis, presented at European Heart Rhythm Association (EHRA) Europace 2013, followed recent NICE guidance supporting safety and effectiveness of subcutaneous implantable defibrillators.
A study is to be the first to prospectively assess the outcomes of pulmonary vein isolation with balloon ablation catheters in patients with persistent atrial fibrillation, according to a company release.
St Jude Medical has announced that is has received CE mark approval of its next-generation quadripolar device, the Quadra Assura MP cardiac resynchronisation therapy defibrillator (CRT-D).
AliveCor has announced that its Heart Monitor for iPhone 4, 4S and 5 is now available for purchase in the UK and Ireland. The device will be on display at the European Heart Rhythm Association Europace 2013 meeting.
In this study, the sleep apnoea monitoring (SAM) algorithm of Sorin Reply 200 pacemakers was validated against polysomnography, the gold standard method used to measure the severity of sleep apnoea.
Model will give physicians more reliable guidance in making therapeutic decisions for stroke prevention.
Andreas Goette is the chair of the European Heart Rhythm Association (EHRA) Europace Scientific Programme Committee. He spoke to Cardiac Rhythm News about his research in molecular electrophysiology, a subject which was absent 15 years ago. He also gave his views on antiarrhytmic drugs and catheter ablation technology, and spoke about the highlights of EHRA Europace 2013.
Researchers in USA and Australia have found a new potentially treatable cause of sudden infant death, consisting of idiopathic ventricular fibrillation preceded by ST segment changes and QRS widening.
Brigitte Osswald
With more than 750,000 new pacemaker implantations and 250,000 first implantable cardioverter defibrillator (ICD) implantations performed worldwide, the relevance of lead extraction strategies becomes vital1.
Prophylactic indications for ICDs and issues like biventricular pacing being in favour of...
Biotronik has announced the European market launch of its Ilesto 7 Series. This is the world's first DF4 implantable cardiac defibrillator (ICD) and cardiac resynchronisation therapy (CRT) series approved for MRI.
A management programme that incorporates a wearable cardioverter defibrillator can be used to bridge a decision for appropriate implantable cardioverter defibrillator (ICD) therapy in patients with acquired, inherited and congenital heart disease, according to 18-month results from the WEARIT-II registry presented at Heart Rhythm 2013
At three-year follow-up, 80% of patients with persistent atrial fibrillation showed freedom from atrial fibrillation and were free from anti-arrhythmic drugs after thoracoscopic ablation with the Bipolar RF Ablation System (AtriCure).
Elderly people may benefit from implantable cardioverter defibrillators as much as younger people, according to new research published in Circulation.
Results from the RELY-ABLE trial, a long-term extension of the pivotal RE-LY study of dabigatran (Pradaxa / Boehringer Ingelheim) in patients with non-valvular atrial fibrillation, have provided additional information showing long-term safety of dabigatran.
Cross-country skiers who take part in one of the world's most challenging ski races, the 90km Vasaloppet in Sweden, are at increased risk of developing cardiac arrhythmias according to a study of nearly 53,000 race participants published online in the European Heart Journal.
The Christ Hospital Health Network, the University of Mississippi Medical Center and Cincinnati Children's Hospital Medical Center have partnered with USRowing and Toshiba to conduct The Athlete Heart Research Study, which aims to determine if sudden cardiac death can be prevented with a heart screening.
St Jude Medical's next-generation devices feature additional safety features to address common lead complications.
A moderate case of obstructive sleep apnoea can significantly increase a person's risk for sudden cardiac death, according to the largest study of its kind published online in the Journal of the American College of Cardiology.
The updated guideline emphasises on patient-centric outcomes such as quality of life, shared decision making, care coordination, transitions, and palliative care.
Results from the OPTION study have shown that patients with dual-chamber implantable cardioverter defibrillators (ICD) experienced a significantly lower incidence of inappropriate shocks compared with patients with standard single-chamber devices (4.3% vs.10.3%, p=0.015).
The multicentre study, published in the June edition of HeartRhythm, is the first to discover that shock-related anxiety is associated with sexual dysfunction in young adults and calls on healthcare providers to address these issues to improve the quality of life for patients.
John D Day is the medical director at Intermountain Heart Rhythm Specialists, Utah, USA. He spoke to Cardiac Rhythm News about his career achievements, the atrial fibrillation ablation programme at the Intermountain Heart Institute, his research on atrial fibrillation and Alzheimer's disease.
Andre d'Avila and Arash Aryana write on three different left atrial appendage strategies aimed to reduce stroke risk in atrial fibrillation patients. d'Avila presented this analysis at Heart Rhythm (Denver, USA, 8-5 May 2013).
At the Heart Rhythm Society (HRS) in Denver, USA, (8-11 May), Vivek Reddy, Mount Sinai School of Medicine, New York, USA, told delegates that leadless right ventricular cardiac pacing is feasible.
The US Food and Drug Administration (FDA) has given conditional approval to Hansen Medical to change the company's study design of its ARTISAN-AF trial.
Serelaxin may be more effective for relieving dyspnoea in heart failure with preserved ejection fraction (HFpEF) than reduced (HFrEF) during the first 24 hours, according to results from RELAX-AHF presented at a late-breaking trial session at the Heart Failure Congress (Lisbon, Portugal, 25-28 May).
Mortality and length of stay are highest in heart failure patients admitted in January, on Friday, and overnight, according to research presented at the Heart Failure Congress 2013 in Lisbon, Portugal (25-28 May).
The IMPACT study aims to investigate whether the use of Biotronik Home Monitoring in conjunction with anticoagulation can reduce the risks of stroke, systemic embolism and major bleeding in cardiac device patients.
A randomised trial (BRUISE CONTROL) has shown that performing device implantation surgery without interruption of warfarin in patients with an annual risk of stroke equal to or over 5% was associated with a significant reduction in device pocket haematoma. Data was presented at the Heart Rhythm Society (HRS) meeting in Denver, USA.
The European Society of Cardiology (ESC) has announced that this years' European Heart Rhythm Association (EHRA) Europace meeting will include, for the first time, late-breaking clinical trial sessions.
Data from the BLOCK HF trial demonstrated that simultaneously pacing the lower chambers of the heart, or biventricular (BiV) pacing with a cardiac resynchronization therapy (CRT) device, significantly improves heart failure symptoms and quality of life in patients with atrioventricular block and left ventricular systolic dysfunction.
A late breaking trial presented at Heart Rhythm 2013 (8-11 May, Denver, USA) has demonstrated that targeted ablation of stable rotors and focal sources can eliminate paroxysmal atrial fibrillation without the need to isolate the pulmonary veins.
On 14 May, UK's National Institute for Health and Care Excellence (NICE) issued updated support for commissioners to help them work with clinicians and managers to commission high-quality, evidence-based anticoagulation therapy for adults across England.
Results from the CITADEL/CENTURION study have shown 95% fewer major cardiovascular implantable electronic device (CIED) infections in patients undergoing CIED replacement procedures using an antibacterial envelope (AIGISRx, Tyrx).
BioControl Medical has introduced a new corporate website that offers patients, family and clinicians an educational resource on its CardioFit therapy for heart failure, as well as the INOVATE-HF clinical study of the device.
Three scientific societies present the first comprehensive recommendations on patients with inherited arrhythmias at Heart Rhythm 2013
The Smartview remote monitoring solution (Sorin) in conjunction with the Sorin Paradym RF device allows valuable cardiac data and alert messages from the device while the patient is at home.
Endosense presented new study data validating the importance of several proprietary contact force parameters in cardiac ablation procedures, including its most recently developed Lesion Index (LSI), at Heart Rhythm 2013.
The Rhythmia Mapping System (Boston Scientific) is a next-generation 3D mapping and navigation solution for use in cardiac catheter ablations and other electrophysiology procedures to treat cardiac arrhythmias including: atrial flutter, atrial fibrillation and ventricular tachycardia.
At HRS, John Cairns, on behalf of the Population Health Research Institute (PHRI), presented five-year follow-up results from an independent analysis of over 10,000 Durata and Riata ST Optim implantable cardioverter defibrillator leads.
GE Healthcare introduced, at HRS, the Innova EPVision 2.0 an application that combines information from EP recording and live fluoroscopy, and Dose Blueprint.
A company release states that this new line of ablation catheters is designed to provide physicians with high resolution, precise, multidimensional information through sophisticated micro-sensors at the tip of the catheter.
On 8 May, St Jude Medical announced the CE mark approval of its next-generation Ellipse and Assura portfolio of implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators (CRT-Ds).
The Training and Credentialing Committee of the Pediatric and Congenital Electrophysiology Society (PACES) has launched a comprehensive review of advanced electrophysiology fellowships in an effort to ensure the highest possible standards for training programmes and practitioners in the field.
Spectranetics has announced that the company will have a major presence to showcase their proprietary laser lead extraction technology and training at the Heart Rhythm 2013 congress in Denver, USA.
A study published in JAMA shows that programming an implantable cardioverter-defibrillator (ICD) with a long-detection interval compared with a standard-detection interval resulted in a reduction in anti-tachycardia pacing episodes, ICD shocks delivered, and inappropriate shocks.
According to a company release, these devices are smaller, thinner and lighter without compromising clinical capabilities.
On 6 May, Medtronic announced the FDA approval and US launch of its newest cardiac devices: the Viva portfolio of cardiac resynchronisation therapy with defibrillation (CRT-D) devices, and the Evera portfolio of implantable cardioverter-defibrillators (ICD).
Studies are exploring renal denervation as an adjunctive treatment in other diseases characterised by sympathetic overactivity such as ventricular arrhythmias, atrial fibrillation, metabolic syndrome and obstructive sleep apnoea. Cardiac Rhythm News spoke to leading physicians who are investigating this technology for the management of atrial fibrillation.
The MultiPoint Pacing study (St Jude Medical) is designed to show improved effectiveness for patients when pacing multiple locations of the heart.
Chen set to begin term as second-ever editor-in-chief of the official journal of the Heart Rhythm Society effective 1 January 2014
Kcentra is the first FDA-approved 4-factor prothrombin complex concentrate for warfarin reversal in the USA.
On 29 April, Hansen Medical announced that it will showcase its Sensei X Robotic Catheter System with the new Artisan Extend Catheter at the Heart Rhythm Society's (HRS) 34th Annual Scientific Sessions (8-11 May, Denver, USA).
The Barostim neo device is an alternative treatment to drug therapy designed to trigger the body's own natural blood flow regulation system to treat heart failure.
Allure Quadra (St Jude Medical) is the first-to-market quadripolar cardiac resincronisation system that allows for increased implant efficiencies that result in fewer surgical revisions, according to a company release.
According to the European Society of Cardiology (ESC), the guide was designed responding to the need to summarise existing information on different drugs, to answer clinical questions that fall outside what drug companies can legally answer, and to make distinctions between the different drugs.
Thomas Vogtmann reviews expert consensus and guidelines on the adoption of remote monitoring, and discusses how clinical practice might change for the better as a result of its increased usage. He gave this talk at the XV International Symposium on Progress in Clinical Pacing in Rome, Italy
Continuous positive airway pressure may help to reduce atrial fibrillation recurrence in patients with obstructive sleep apnoea undergoing pulmonary vein isolation, a study published ahead of print in the Journal of the American College of Cardiology (JACC) has found.
Cardiovascular scientists from the University of Michigan, USA, and St George's University of London, UK, announced the launch of avert-AF, a new initiative aimed at developing drug treatments for the prevention of the more advanced forms of atrial fibrillation.
On 22 April, the board of trustees of the American College of Cardiology (ACC) announced that Shalom "Shal" Jacobovitz has been selected as the college's chief executive officer.
A new treatment using shock wave (procedure using high-dose ultrasound) and mononuclear cells was associated with significant, albeit modest improvement in left ventricular ejection fraction (LVEF) after four months in patients with chronic postinfarction heart failure.
Left anterior fascicular block may be a clinically relevant marker of an individual's propensity to develop atrial fibrillation or congestive heart failure, a new study has found.
The first patient has been implanted with the next generation ImageReady MR Conditional pacing system (Boston Scientific) in the SAMURAI clinical trial.
On 15 April, TYRX announced that the first ever implantation of its new AIGISRx R Fully Resorbable Antibacterial Envelope took place at the Quebec Heart and Lung Institute in Quebec City, Canada by François Philippon.
CardioFocus has announced that presentations of the HeartLight system and its role in the treatment of advanced atrial fibrillation were featured at the 79th Annual Meeting of the German Cardiac Society in Mannheim, Germany.
Spectranetics has announced that the lead extraction simulator, a virtual, hands-on environment to train physicians in the removal of pacemaker and defibrillators leads, will be taken directly to the physicians and the hospitals where they practice with a fully mobile version.
The topics include home discharge using the Freedom Portable Driver and the use of the Total Artificial Heart for adolescents, congenital patients and destination therapy.
Inliven (Boston Scientific) is the first cardiac resynchronisation therapy pacemaker (CRT-P) using patient's respiration signals to provide physiological pacing and comorbidity management.
The system helps physicians determine the most appropriate location for left-ventricular lead placement by generating 3-D images of the cardiac veins.
Results from the EFFICAS I, a prospective multicentre study sponsored by Endosense, have led to the development of guidelines for target and minimum contact force, as well as minimum force time integral, during the catheter ablation treatment of paroxysmal atrial fibrillation.
According to a company release, Iforia is the world's first DF4 ICD/CRT-D series approved for magnetic resonance imaging.
Approval by the Japanese Pharmaceuticals and Medical Devices Agency follows a successful three-year clinical trial of the Niobe remote magnetic technology for cardiac ablations at the Tokyo Women's Medical University.
The FDA announced that if the proposed order is finalised it will require manufacturers of these life-saving devices to submit pre-market approval applications.
Preliminary results of the PREVAIL trial have demonstrated the Watchman device (Boston Scientific / Atritech) is a safe alternative to oral anticoagulation therapy for stroke prevention in non-valvular atrial fibrillation patients. However, there are now questions towards Watchman's efficacy.
A small study presented at ACC 2013 has shown that renal denervation, besides reducing resistant hypertension, produces a favourable effect on atrial and ventricular arrhythmias.
Cardiac Rhythm News speaks to Mark S Kremers, chairman, ICD Registry Steering Committee, on the results from the latest US ICD registry led by the Heart Rhythm Society and the American College of Cardiology Foundation. In this registry, Kremers said: "about 90% of all ICDs implanted in the USA are tracked".
The trial will study the safety and effectiveness of the SonR cardiac resynchronisation therapy (CRT) optimisation system in patients with advanced heart failure.
There are many concerns on leadless technology. However, with the increase recognition of lead related problems and advantages of endocardial pacing, leadless technology will be the next frontier of cardiac pacing. Chu-Pak Lau, Hong Kong, writes for Cardiac Rhythm News.
The updated registry, developed through a partnership of the Heart Rhythm Society and the American College of Cardiology Foundation, captures lead data and paediatric procedures for the first time.
The FDA has issued a Drug Safety Communication as a result of its review of a study by medical researchers as well as another study by Pfizer that assessed the potential for azithromycin to cause abnormal changes in the electrical activity of the heart.
In parallel with the American College of Cardiology (ACC) Scientific Sessions in San Francisco, USA, initial results have been released from a physician educational programme being undertaken to rectify misunderstandings on stroke prevention in atrial fibrillation in China.
Even minor weight loss is associated with worse health outcomes among patients implanted with cardiac resynchronisation therapy with defibrillator (CRT-D), according to research presented by Valentina Kutyifa, Rochester, USA, at ACC 2013.
Robert S White is president and CEO of TYRX, the company behind the AIGISRx Antibacterial Envelope, which is designed to reduce surgical site infections associated with cardiac implantable electronic devices.
Zio Patch, anambulatory cardiac monitoring system (iRhythm), has shown benefit of long-term monitoring or paroxysmal atrial fibrillation patients and significant impact on treatment strategy.
The ACP (Amplatzer Cardiac Plug) clinical trial is designed to determine if the Amplatzer device (St Jude Medical) is safe and effective in preventing thrombus from migrating out of the left atrial appendage in patients with non-valvular atrial fibrillation who have a high risk for stroke.
Researchers in the United Kingdom have found in a first-of-its-kind study that fibrosis detected by late gadolinium enhancement cardiac MRI may be an independent predictor of hard cardiovascular events in patients with ventricular arrhythmias.
Massimo Santini, Rome, Italy, and others have recently published results from the PRESS study. They found that by using a dual chamber pacemaker programmed to 60ppm lower rate the number of syncope events can be reduced in bifascicular block patients.
Boston Scientific will present, for the first time, preliminary results of all three co-primary endpoints of the Watchman device at the American College of Cardiology 2013 Annual Scientific Sessions.
People who suffer from insomnia appear to have an increased risk of developing heart failure, according to a study published online in the European Heart Journal.
The added finances will further support Endosense's programme to get access to the US market with its TactiCath Quartz contact-force sensing ablation catheter and to reinforce sales in Europe.
JACC: Heart Failure is a new journal dedicated to meet the demands and challenges of treating what is now the most rapidly increasing category of cardiac disease. The first publication is now available.
The Annual Frankfurt Atrial Fibrillation Academy Symposium will highlight the latest innovations and research in atrial fibrillation therapy.
The Attain Performa leads (Medtronic) are designed with shorter spacing between the two centre electrodes to increase phrenic nerve thresholds and reduce phrenic nerve stimulation, a company release states.
The document, developed by the American College of Cardiology, the Heart Rhythm Society and other key specialty societies, examines potential clinical scenarios to support physician decision making.
"Ventricular fibrillation was generally viewed as a non-ablatable rhythm disturbance. However, several advances over the last decade have enabled electrophysiologists to successfully address this arrhythmia with ablation techniques," writes Samuel J Asirvatham, Mayo Clinic, Rochester, USA.
ISIS-CRP(Rx) is being evaluated in patients with atrial fibrillation to measure the effect of lowering C-reative protein on the frequency and duration of the episodes.
Alcard is a Brazilian leading manufacturer of medical devices for cardiac surgery.
On 27 February, the UK's National Institute for Health and Clinical Excellence (NICE) released final guidance recommending apixaban (Eliquis, Bristol-Myers Squibb and Pfizer) as an option for the prevention of stroke and systemic embolism in some people with non-valvular atrial fibrillation.
The Lumax 740 DX System from Biotronik expands the diagnostic capabilities of a standard single-chamber ICD with a single lead, in addition to featuring sophisticated sensors that allow for atrial monitoring and enhanced arrhythmia diagnosis.
Richard Hongo, cardiac electrophysiologist, California Pacific Medical Center, San Francisco, USA, explains how ablation of ventricular tachycardias works.
https://youtu.be/Y-YzXbfcAas
According to a Massachussetts General Hospital press release this is one of the only institutes in the world to integrate cardiovascular and cerebrovascular care.
According to study results of the Advisa MRI System clinical study, the system, recently approved by the FDA, allows magnetic resonance imaging access without compromising positioning restrictions, including the cardiac region.
A new sub-analysis of the MADIT-CRT multicentre trial has shown that implantable cardioverter defibrillator (ICD) shocks at the time of defibrillation threshold testing in patients with mild symptoms of heart failure are not associated with an increased risk of heart failure or death or future episodes of ventricular tachycardia and ventricular fibrillation.
Despite the advent of non-fluoroscopic technology, fluoroscopy remains an imaging cornerstone, both in interventional electrophysiology and in device implantation. Moreover, many patients receive additional X-ray imaging, such as cardiac CT and others. Hein Heidbuchel outlines a new European Heart Rhythm Association (EHRA) guide to radiation protection.
The AirStrip mobility solution will deliver live patient data from CardioNet's Mobile Cardiac Outpatient Telemetry (MCOT) directly to clinicians' mobile devices, including tablets and phones.
The Ilesto 7 series ( Biotronik) includes one of the world's smallest ICDs and features ProMRI technology, which enables access to potentially life-saving MR scans.
The Heart Rhythm Society calls on all Americans to know risks and warning signs of the most common arrhythmia during February the Heart Health Month.
The ELI 280 is a 12-lead resting electrocardiograph featuring a tablet-style touch screen user interface .
The Advisa MRI system is Medtronic's second-generation MR-Conditional pacemaker and, according to a company release, is the first system to combine the most advanced pacing technology with proven magnetic resonance imaging (MRI) access.
The Scottish Medicines Consortium has accepted the novel oral anticoagulant apixaban (Eliquis, Bristol-Myers Squibb / Pfizer) for use within NHS Scotland for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors.
Vivek Y Reddy, Mount Sinai School of Medicine, New York, USA, and others recently reported the updated results ahead of print in Circulation.
Researchers in The Netherlands have found that there is a borderline significant association between statin therapy and depression and statin therapy and physical functioning in patients with an implantable cardioverter defibrillator.
The Heart Rhythm Society has several campaigns to raise awareness of heart rhythm disorders, such as "Arrest the Risk Awareness" that aims to raise awareness of sudden cardiac arrest among black and Hispanic communities. Anne Gillis reviews the importance of patient education.
The new fully automatic unit provides all the same Full-Rescue features and benefits as the semiautomatic version of the AED Plus except that a shock is delivered automatically if one is advised.
The Heart Rhythm Society and the Association of Black Cardiologists are working in conjunction to help reduce the incidence of sudden cardiac arrest among African Americans through a new campaign titled 10-City Arrest the Risk.
Now with the help of Rubio, the HeartRescue Project is unveiling the latest life-saving innovation, an interactive online experience called the "Save-A-Life Simulator".
Janssen Pharmaceuticals in conjunction with Portola Pharmaceuticals and Bayer HealthCare will evaluate several dosage strengths of PRT4445 and its ability to reverse the anticoagulant activity of rivaroxaban in emergency situations.
GE Healthcare has announced two new software packages for advanced analysis of cardiovascular magnetic resonance (MR) images: CardiacVX and MR VesselIQ Xpress.
The MoniC study, conducted at the Campus Charite Mitte, Berlin, addressed the feasibility, safety, workload and clinical usefulness of a centralised Biotronik Home Monitoring model.
The new Evera ICD portfolio (Medtronic) features increased longevity and advanced shock reduction technology.
The first US case was performed on 31 January 2013 as part of an amended protocol of the company's TOCCASTAR investigational device exemption clinical study of the TactiCath called the TOCCASTAR Supplemental Clinical Study.
A first-of-its-kind study by Dhanunjaya Lakkireddy, Kansas, USA, and others has found that rigorous practice of yoga can help reduce episodes of irregular heartbeat and improve the symptoms of anxiety and depression often associated with atrial fibrillation.
The new envelope includes the advantages of the original AIGISRx, such as stability and implantable electronic devices' infection reduction, but now with the added benefit of being fully resorbable.
A large population-based study from Finland, published in the European Journal of Preventive Cardiology, has shown that being unmarried increases the risk of fatal and non-fatal heart attack in both men and women whatever their age.
GENETIC-AF is projected to be a 620-patient, phase 3 study comparing Gencaro (ARCA biopharma) to metoprolol CR/XL for prevention of atrial fibrillation in patients with heart failure and left ventricular dysfunction.
ZOLL Medical has announced that ZOLL is the first major defibrillator manufacturer to publicly pledge the sharing of patient data to reduce preventable deaths.
Dabigatran (Pradaxa, Boehringer Ingelheim) was the first of three novel anticoagulants, the others being rivaroxaban (Xarelto, Bayer) and apixaban (Eliquis, Bristol Myers Squibb, Pfizer), to be approved for stroke prevention in patients with non-valvular atrial fibrillation. Giuseppe Di Pasquale reviews the use of the drug in clinical practice.
Faster lower pacing rate immediately after atrioventricular node ablation with a gradual decrease helps to improve clinical outcome and may reduce the risk of sudden death in patients with atrial fibrillation, Ru-Xing Wang and other reported in Heart Rhythm ahead of print.
These external defibrillators feature a revolutionary color LCD video display and 3-lead ECG monitoring capability.
MATRIX is a prospective open study that will include 2,000 patients in 100 sites throughout Europe, Latin America, Canada, Israel and Japan for up to four years.
On 23 January, the UK's National Institute for Health and Clinical Excellence (NICE) issued a final draft guidance recommending apixaban (Eliquis, Bristol-Myers Squibb and Pfizer), in accordance with its licensed indications, for the prevention of stroke and systemic embolism in some people with non-valvular atrial fibrillation.
Pasquale Santangeli, Texas Cardiac Arrhythmia Institute, St David's Medical Centre, Austin, USA, spoke to Cardiac Rhythm News about the link between atrial fibrillation and dementia.
Gregory YH Lip, Birmingham, UK, talked to Cardiac Rhythm News about the highlights of his career, including his mother as his biggest influence, and the recent update to the European Society of Cardiology (ESC) guidelines for the management of atrial fibrillation.
Study data suggest it is safe to use CorMatrix ECM technology to close the pericardium of first-time coronary artery bypass graft patients.
The Amulet device is used to close the left atrial appendage (LAA) in patients diagnosed with non-valvular atrial fibrillation.
This next generation of technology includes sensors along the ablation device which provide real-time feedback to physicians conducting cardiac ablation procedures.
Philips provides a novel approach to real-time 3D imaging with improved workflow and collaborates with Biosense Webster to provide enhanced anatomical detail in the Biosense Carto 3 mapping system.
The new catheter provides real-time, high-resolution ultrasound images of blood flow and cardiac anatomy when paired with the ViewMate Z Intracardiac Ultrasound Console.
The ZERO AF study will include up to 33 sites in the USA, Europe and Asia-Pacific, and the results will be used to support a FDA submission for a paroxysmal AF indication.
The ProMRI trial will evaluate Biotronik's Entovis dual- and single-chamber pacemaker systems after exposure to magnetic resonance imaging at 30 US investigational centres.
The newest version of Merlin.net provides clinicians with additional insight into changes in their patients' heart failure status through advanced impedance and lead monitoring capabilities.
Medtronic has announced the first patient implant for the REVEAL AF trial, which will evaluate the incidence of AF among patients suspected to be at a high-risk for the disease.
New NICE medical technologies guidance published on 16 January 2013 supports the use of a device that can detect atrial fibrillation whilst blood pressure is being measured.
The fully automatic external defibrillator system, which has been developed by Defibtech, was designed to provide a shock automatically when a heart arrhythmia is detected.
The HeartLight pivotal trial is randomising the HeartLight system against the ThermoCool Catheter, and will randomise an estimated 350 patients in the multicentre US trial.
The FlexCath Advance Steerable Sheath is a new enhancement to the Arctic Front Advance Cryoballoon System, which is expected to facilitate easier access to the inferior veins when treating paroxysmal atrial fibrillation.
CardioFocus has announced that more than 1,000 clinical cases have been performed worldwide using its HeartLight Endoscopic Ablation System technology for the treatment of atrial fibrillation.
The trial seeks to evaluate the effectiveness of cardiac resynchronisation therapy-pacemakers (CRT-Ps) in symptomatic heart failure patients with mild-to-moderate structural heart disease.
On 28 December 2012, the FDA approved the anticlotting drug apixaban (Eliquis, Bristol-Myers Squibb), an oral tablet used to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation.
One year after treatment completion, 80% of patients in normal sinus rhythm without administration of drugs, study says.
Boston Scientific is beginning a clinical trial of patients implanted with the INGEVITY device.
Nanostim announces the implantation of its leadless pacemaker system in the LEADLESS study.
ECOST trial results published in European Heart Journal show the safety and efficacy of Home Monitoring.
The oral anticoagulant is a new prescription only treatment option for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation and one or more risk factors such as: prior stroke or transient ischaemic attack; age 75 years or older; hypertension; diabetes mellitus; symptomatic heart failure (NYHA Class ≥ II).
BioControl Medical has announced that the pilot clinical study of its CardioFit vagus nerve stimulation system has been recognised as seminal original research in the European Journal of Heart Failure.
Findings from the RE-LY trial and four other phase III trials report outcomes after a major bleed on dabigatran, despite the lack of a specific reversal agent, may be better than after a warfarin-associated bleed.
Marsault joins the company as it continues to expand the commercial presence of its TactiCath contact-force sensing ablation catheter in Europe and prepares for the device's anticipated launch in the United States.
According to a study published in the Journal of the American College of Cardiology, a 20-year ECG screening programme of young athletes would only save 4,813 lives and would cost between US$51 billion and US$69 billion-resulting in enormous costs per life saved.
A study recently published in EP Europace has shown that successful catheter ablation of atrial fibrillation may reduce the risk of total mortality, cardiovascular mortality and total vascular events in patients with an increased risk of stroke.
Syncope is a very common medical problem with a yearly incidence of around 10 patients per 1,000 inhabitants. However, the rate of diagnosis when using a non-standardised approach is 42-54%. Mohamed Hamdan reviews the evidence for syncope units and whether they can be used to improve the rate of diagnosis of syncope.
The Smartview remote monitoring system enables the patient's implantable device to communicate stored data wirelessly to the clinic where clinicians can review the information via a secure web application.
The Heart Monitor is initially intended for use by licensed medical professionals to record, display, store, transfer, and evaluate single-channel electrocardiogram rhythms.
The intracardiac impedance sensor evaluates changes in impedance during the cycle of the heart and is expected to be a useful surrogate for several left ventricular haemodynamic parameters.
A study published in the Journal of the Neurological Sciences has found that Mobile Cardiac Outpatient Telemetry (MCOT) supports the detection of a high rate of occult paroxysmal atrial fibrillation (PAF) in cryptogenic stroke and transient ischaemic attack (TIA) patients.
The Assura devices including the Quadra Assura CRT-D, the Unify Assura CRT-D and the Fortify Assura ICD, all from St Jude Medical, feature three new algorithms, more power and extended longevity.
Proceeds will be used to support commercialisation of the recently European introduction of the third-generation TactiCath Quartz force-sensing catheter; development of next generation products; completion of the TOCCASTAR investigational device exemption clinical study follow-up and FDA approval submission for the TactiCath.
HeartSine has notified that certain Samaritan 300/300P public access defibrillators (PAD) have been found to intermittently turn on and off, which may eventually deplete the battery.
Wolfram Grimm, Baldingerstraβe, Germany, and others reported in EP Europace that while undiagnosed sleep-disordered breathing is common in patients with implantable cardioverter defibrillators (ICD) and chronic heart failure, it does not independently predict appropriate ICD therapy or mortality.
At Europe AF (18-19 November, London, UK), Dirk Mueller, Bad Bevensen, Germany said that the initial results of the GENESIS study validated the usability of the Over-the-Wire Mesh Ablation System (OTW MAS, Encompass, Bard Electrophysiology) for paroxysmal atrial fibrillation.
The European approval for apixaban is supported by the pivotal phase 3 trials ARISTOTLE and AVERROES, which evaluated approximately 24,000 patients with non-valvular atrial fibrillation.
Boston Scientific has received the CE mark approval for increased longevity projections for the Incepta, Energen, Punctua, Cognis and Teligen implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators (CRT-Ds).
Evia HF-T offers Biotronik's Closed Loop Stimulation (CLS) technology. The company states that this is "the only rate response algorithm that is FDA-approved to respond to exercise and acute mental stress on a beat-to-beat basis."
New data have shown that acute pulmonary vein isolation was achieved after a single visually guided circular lesion set in 89% and 69% of patients in the high and low-dose groups, respectively.
According to a company release, the HeartStart FRx AED has been tested and certified for airline use and does not interfere with airplane electronics.
The Population Health Research Institute analysed data from three actively monitored registries: the OPTIMUM, SCORE and SJ4 Post-Approval registries, all sponsored by St Jude Medical. The combined data from these registries currently represents 10,987 leads implanted at 293 sites.
Use of the new code allows the German Institute of Medical Documentation and Information to track the number of atrial fibrillation patients being treated with laser ablation guided by endovascular endoscopy and provides the initial step to receiving a specific diagnosis-related group code for the procedure.
New data from the RELY-ABLE study have provided additional support to the safety profile and efficacy of dabigatran etexilate (Pradaxa, Boehringer Ingelheim) for stroke prevention in patients with non-valvular atrial fibrillation.
Piezoelectric harvesters could be used to convert heartbeat-induced vibrations into enough electrical energy to power a cardiac implantable electronic device, according to a preliminary study presented by M Amin Karami, Ann Arbor, USA, at the scientific sessions of the American Heart Association in Los Angeles.
Jerrett Lau, Concord Hospital, Sydney, Australia, and others reported, at the American Heart Association (AHA) scientific sessions, that a smartphone app could be used to diagnose atrial fibrillation in a community setting.
Data presented at the American Heart Association's 2012 Scientific Sessions show 26% relative risk reduction in the composite of death, healthcare utilisation visits requiring IV heart failure therapy, and significant increase in left ventricular end systolic volume index among the patients receiving biventricular pacing.
Data presented at the American Heart Association Scientific Sessions have shown that improved programming of dual-chamber implantable cardioverter defibrillators or cardiac resynchronisation therapy defibrillators can reduce inappropriate therapy by up to nearly 80% and increase patient survival by up to 55%.
Results from the GOLDEN TIME study have shown that gold-tip radiofrequency ablation catheters enable faster, more efficient ablation procedures than platinum-iridium-tip catheters.
The assessment is consistent with observations from the large RE-LY clinical trial used to approve dabigatran (Pradaxa) for the prevention of stroke in patients with non-valvular atrial fibrillation.
A new Lancet Series explores the latest developments in the diagnosis, treatment and biology of cardiac arrhythmias, ahead of the American Heart Association's annual meeting (Los Angeles, USA, November 3-7).
Michael H Carrel was most recently the president and CEO of Vital Images. Under his leadership at Vital Images, the company grew revenue and profitability, increased global market share, expanded its presence to over 90 countries and raised US$100 million in equity financing.
Sudden cardiac arrest (SCA), commonly mistaken for a heart attack, is one of the leading causes of death in the United States each year, and approximately 95 percent of people who experience the condition die as a result. The...
Implantable devices for treating cardiac arrhythmias, which include ICD's, are underused in parts of Europe. Conclusions of the ICD for Life Summit (Belgrade, Serbia, 19-20 October).
With the Advisa MRI system, pacemaker patients in Japan will have access to full body scans, without positioning limitations in the magnetic resonance imaging (MRI) scanner.
Randy Lieberman, director of electrophysiology at Harper University Hospital: CardioVascular Institute, Detroit Medical Center, Detroit, USA, reviews the role of the parasympathetic nervous system in heart failure.
Behzad Pavri, Philadelphia, USA, and colleagues have found that implantable cardioverter defibrillators (ICDs) with three or more years of estimated remaining battery life can be reused, delivering life-saving therapy without increasing the risk of complications.
Krzysztof Bartus, Krakow, Poland, and colleagues have reported that left atrial appendage closure for stroke prevention in patients with atrial fibrillation using a percutaneous ligation approach can be performed effectively and is associated with a low rate of complications.
The four patents issued cover essential components of the multidisciplinary epicardial-endocardial approach pioneered by nContact for the treatment of cardiac arrhythmias.
The survey has shown that 75% of Americans are unaware that an implantable cardioverter defibrillator (ICD) is an effective treatment option to protect those at risk of SCA.
The device is a subcutaneous implantable leadless cardiac monitor for the long-term continuous remote monitoring of patients with arrhythmias such as atrial fibrillation (AF), bradycardia, sudden rate drop, asystole and tachycardia.
Data show high acute success and freedom from atrial fibrillation at six months follow-up.
Rhythmia Medical is a developer of next-generation mapping and navigation solutions for use in cardiac catheter ablations and other electrophysiology procedures, including atrial fibrillation and atrial flutter.
Stephen Page and Richard Schilling, St Bartholomew's Hospital, UK, explain why catheter ablation can be performed safely in most patients with uninterrupted warfarin.
Yan Liang, Beijing, China, and colleagues have found that moderate alcohol consumption may increase the risk of atrial fibrillation among people with existing cardiovascular disease and that binge drinking further increases this risk. The researchers analysed data from 30,433 patients enrolled in the ONTARGET and TRANSCEND trials.
A study, published ahead of print in Circulation Arrhythmia and Electrophysiology, has found that inappropriate programmed atrioventricular delays was the most common cause of sustained loss of CRT pacing.
The Cardio7 is a 12 channel interpretive resting ECG with a 7" hi-resolution color TFT LCD touch screen.
The new drug application for apixaban (Eliquis / Bristol-Myers Squibb and Pfizer) is intended to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation.