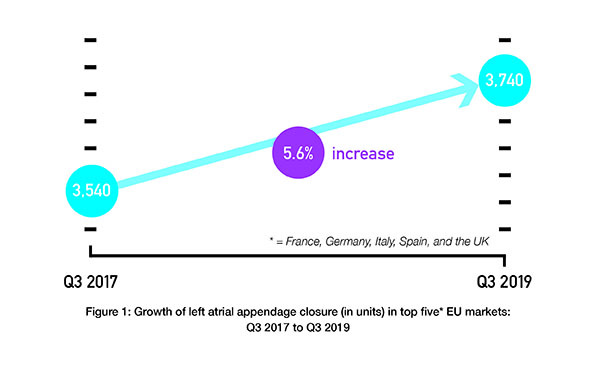
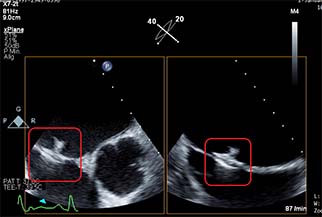
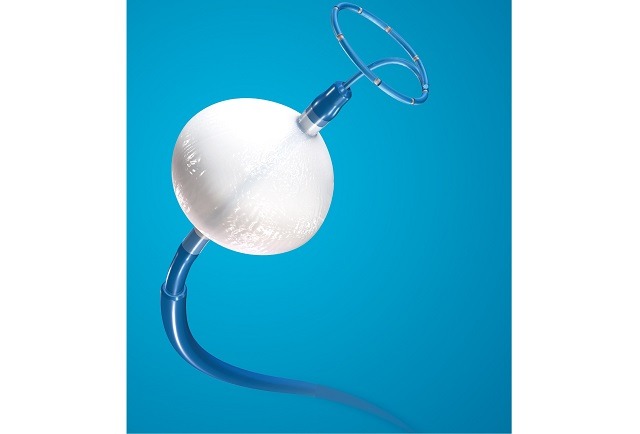
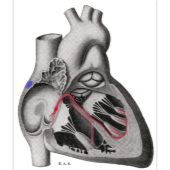
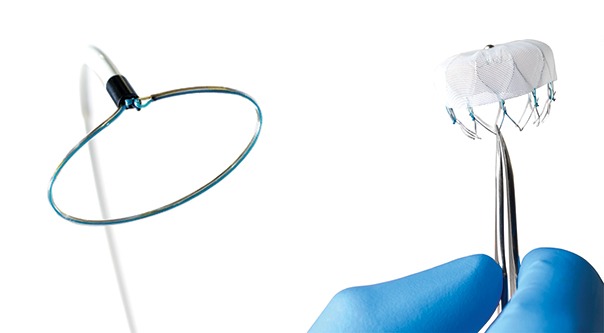
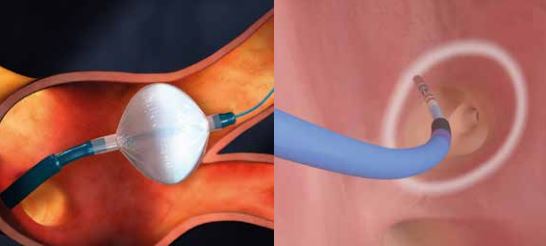
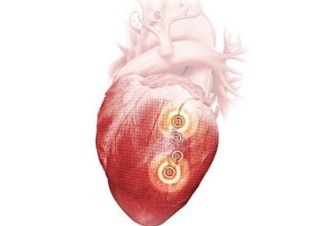
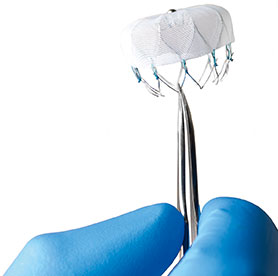
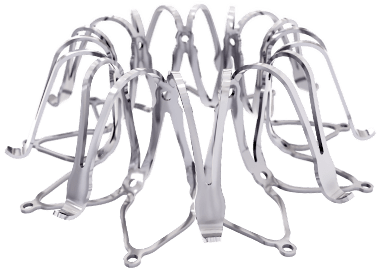
Left atrial appendage occlusion (LAAO) improves clinical outcomes including ischaemic stroke, major bleeding and all-cause mortality, in atrial fibrillation (AF) patients with a high risk of stroke and major bleeding, compared to the use of novel oral anticoagulants (NOACs)....
Patients with COVID-19 who were admitted to an intensive care unit were 10 times more likely than other hospitalised COVID-19 patients to suffer cardiac arrest or heart rhythm disorders, according to a new study from researchers in the Perelman...
Royal Philips has announced that the Center for Devices and Radiological Health (CDRH) of the US Food and Drug Administration (FDA) has granted premarket approval (PMA) for the company’s HeartStart FR3 and HeartStart FRx automated external defibrillators (AEDs), and...
Voice analysis by a smartphone app can identify lung congestion in heart failure patients, allowing early intervention before their condition deteriorates, a study presented on HFA Discoveries, a scientific platform of the European Society of Cardiology (ESC) has found.
“Speech...
Proximo Medical has announced a partnership with digital health company Coala Life in select US markets. According to a press release from Proximo Medical, it provides a business acceleration solution to addresses medical device commercialisation challenges among startup and...
Medtronic has received CE mark for Micra AV Transcatheter Pacing System (TPS), the world’s smallest pacemaker with atrioventricular (AV) synchrony.
Micra AV is indicated for the treatment of patients with AV block, a condition in which the electrical signals between...
CardioFocus has announced that the first patients have been treated commercially with the recently-approved HeartLight X3 endoscopic ablation system in the USA. The cardiac ablation technology is designed to treat drug refractory, symptomatic paroxysmal atrial fibrillation (AF), the most...
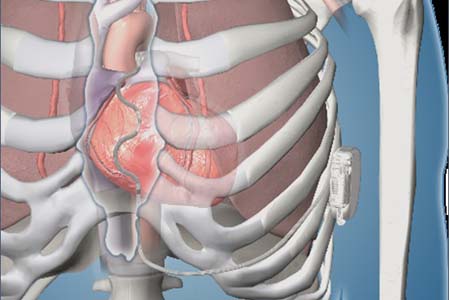
Biotronik has announced its commitment to giving physicians additional tools to pace in the His-Bundle, coinciding with the launch of its His-Bundle Pacing (HBP) tools in a limited number of centres with a full launch later in 2020. The...
Jeffrey L Turner, Brian Zenger and Benjamin Steinberg (all University of Utah School of Medicine, Salt Lake City), write that social media has become a hub of activity for clinical electrophysiologists (EPs) to exchange clinical and scientific material, the...
As part of this year’s HRS 2020 Science virtual event Douglas L Packer (Mayo Clinic, Rochester, USA) delivered the 2020 Eric N Prystowsky lecture on lessons learned from the CABANA trial.
Packer described the lecture as a discussion on “what...
A joint document has been issued by the Heart Rhythm Society (HRS), the American Heart Association (AHA), and the American College of Cardiology (ACC) to provide guidance for clinicians and institutions on re-establishing safe electrophysiological (EP) care.
Published in Heart...
Global cardiac health societies have collaborated on a consensus statement on the diagnosis of arrhythmias, designed to provide physicians with practical proposals to improve patient care in the field.
The international consensus statement on risk assessment in cardiac arrhythmias was...
Researchers have used an optical mapping system to observe how the malaria drug hydroxychloroquine, which has been touted as a possible treatment for COVID-19, creates disturbances in heart rhythm in an animal model. The research, published in the journal...
Adagio Medical has received CE mark approval for its ultra-low temperature intelligent Continuous Lesion Ablation System (iCLAS) for the endocardial treatment of paroxysmal (PAF) and persistent (PsAF) atrial fibrillation and atrial flutter.
“More than half of all atrial fibrillation (AF)...
The British Heart Foundation (BHF) is calling on the Government and the NHS to urgently address the immediate needs of heart and circulatory patients who have had care postponed during the coronavirus pandemic. The charity made its statement after...
As electrophysiologists worldwide begin the process of restarting elective procedures put on hold due to the COVID-19 pandemic, cardiology departments are braced for a wave of “forgotten” patients whose conditions may have worsened over time.
This was among the...
Abiomed has received investigational device exemption (IDE) from the US Food and Drug Administration (FDA) for an early feasibility study with a first-in-human trial of Impella ECP (expandable cardiac power), a 9 French (Fr) heart pump. It will be...
The American College of Cardiology (ACC) is to host a weekly virtual meeting— the Summer COVID-19 Education Series—to provide the healthcare community with actionable insights and solutions that will address key clinical and operational concerns and challenges during the...
The European Society of Cardiology (ESC) has announced that the HFA Discoveries online event will be running six days of live sessions from 5 to 19 June, looking at prevention and treatment strategies in heart failure. The sessions will...
Boston Scientific has announced the US launch of DirectSense technology, a tool for monitoring the effect of radiofrequency (RF) energy delivery during cardiac ablation procedures. According to a press release, the DirectSense technology, which received US Food and Drug...
Highlights:
Cardiologists discuss the "return to normal" as COVID-19 restrictions ease
Late breaking trials from HRS 2020 Science including: PRAETORIAN, UNTOUCHED and PINNACLE FLX
Profile: Athena Poppas
Malcolm Finlay discusses the growing role of artificial intelligence in the diagnosis...
Bardy Diagnostics has received CE mark certification for the 14-day version of the Carnation Ambulatory Monitor (CAM) patch, the p-wave centric ambulatory cardiac patch monitor and arrhythmia detection device. The 14-day CAM patch provides clinicians greater flexibility tomonitor their...
The 2020 edition of the Heart Rhythm Congress (HRC 2020), which had been scheduled to take place 27–30 September in Birmingham, UK, has been moved to an online platform.
Organisers have taken the decision to host the meeting online, as...
A study into the use of hydroxychloroquine or chloroquine, published in The Lancet, has failed to find a benefit in hospital-outcomes from the use of the drugs for the treatment of COVID-19. The study also associates the drug with...
Play the same piece of music to two people, and their hearts can respond very differently. That is the conclusion of a study presented on EHRA Essentials 4 You, a scientific platform of the European Society of Cardiology (ESC)....
Eko has announced the launch of Eko Telehealth, an artificial intelligence (AI)-powered telehealth platform for virtual cardiac and pulmonary monitoring. Being used by more than 200 health systems today, Eko Telehealth incorporates stethoscope and ECG live-streaming combined with embedded...
Acutus Medical and Biotronik have announced a new alliance to provide a comprehensive portfolio of electrophysiology, mapping, ablation and accessory products for catheter-based treatment of cardiac arrhythmias across select markets, including Europe and Asia.
At the centre of the new...
Cardiologs, in partnership with the Valley Health System, has announced the results of a clinical study that found the Cardiologs AI-based ECG analysis solution reduces by almost 70% the false positives of atrial fibrillation (AF) detected by implantable loop...
Thermedical has announced that results from a first-in-human early feasibility study (EFS) using the Durablate catheter to treat ventricular tachycardia (VT) were presented in the late-breaking clinical trial sessions at the Heart Rhythm Society (HRS) 2020 Science online meeting.
Titled...
The American College of Cardiology (ACC) is to collect COVID-19 data through the NCDR Chest Pain-MI and CathPCI registries with the aim of capturing the relationship between COVID-19 and heart disease and maximise data on the cardiac impact of...
Royal Philips has announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) to market a wide range of its ultrasound solutions for the management of COVID-19-related cardiac and lung complications.
Handheld and portable ultrasound...
David E Albert, founder and chief medical officer of AliveCor, considers the impact of the COVID-19 pandemic on the monitoring and of management of arrhythmias.
As the world continues to fight against the COVID-19 pandemic, healthcare providers and professionals are...
The US Food and Drug Administration (FDA) has expedited clearance of an update to software from Caption Health, a medical artificial intelligence (AI) company, that is designed to aid frontline healthcare workers to perform diagnostic-quality cardiac ultrasounds.
Following a...
CardioFocus has announced that the US Food and Drug Administration (FDA) has approved the next-generation HeartLight X3 endoscopic ablation system for the treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation (PAF).
Approval of the HeartLight X3 system came as...
Medtronic has received US Food and Drug Administration (FDA) approval for its Cobalt and Crome implantable cardioverter-defibrillators (ICD) and cardiac resynchronisation therapy-defibrillators (CRT-D).
ICDs monitor heart rhythms and deliver therapy to correct heart rates that are too fast and can...
Corresponding author Valentin Fuster (Mount Sinai Heart, New York, USA) and colleagues report in the Journal of the American College of Cardiology that hospitalised COVID-19 patients treated with anticoagulants had improved outcomes both in and out of the intensive...
AtriCure has announced results from the CONVERGE IDE clinical trial, which showed an 18% difference in favour of the hybrid Convergent procedure as compared to endocardial catheter ablation in the treatment of atrial fibrillation (AF). The results of the...
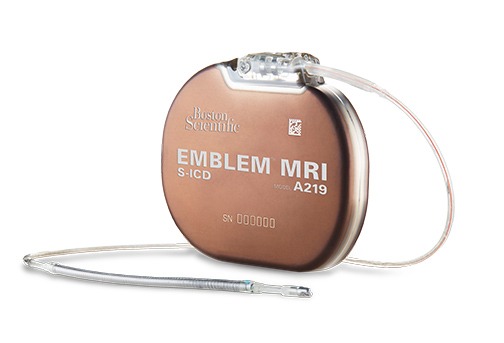
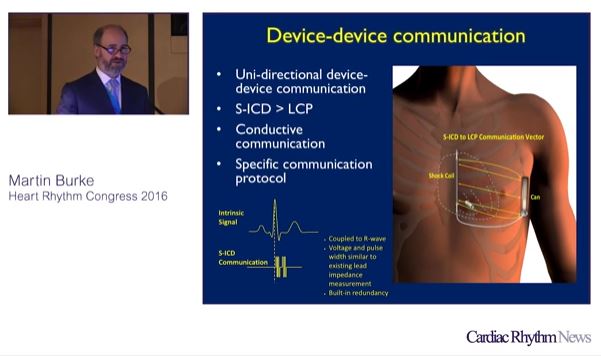
Findings from the UNTOUCHED study, presented online in a late-breaking trial session at HRS 2020 Science, showed that patients receiving subcutaneous implantable cardioverter defibrillator (S-ICD) therapy experienced a higher inappropriate shock-free rate than in previous S-ICD and transvenous implantable...
Medtronic has announced results from late breaking clinical trials, presented online at HRS 2020 Science, evaluating the MyCareLink Heart mobile app and the Micra Transcatheter Pacing System (TPS).
Results from the BlueSync Evaluation study demonstrated that patients who used the...
Contact force (CF) sensing catheters have been shown to be safe and effective in persistent atrial fibrillation (AF) ablation, according to the findings of the PRECEPT study, which have been presented online during a late-breaking clinical trial session at...
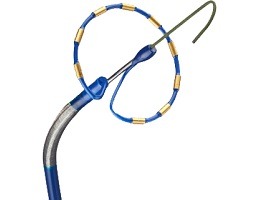
Boston Scientific has announced positive 12-month results from the PINNACLE FLX clinical trial assessing the safety and efficacy of the next-generation WATCHMAN FLX Left Atrial Appendage Closure (LAAC) Device for patients with non-valvular atrial fibrillation (NVAF). Presented as a...
Subcutaneous implantable cardioverter defibrillators (S-ICDs) are as protective as transvenous implantable cardioverter-defibrillators (ICDs) in the prevention of sudden cardiac death, but have a better safety profile, the results of a prospective, randomised trial online in a late-breaking trial session...
Initial results of the PULSED AF trial, the first-in-human results for paroxysmal or persistent atrial fibrillation (AF) treated with pulsed field ablation (PFA) were presented online as part of a late breaking clinical trial session at Heart Rhythm 2020....
The Heart Rhythm Society (HRS) has released a white paper announcing the rationale and core components for Atrial Fibrillation (AF) centres of excellence (CoE), to provide multidisciplinary care for AF patients. The goal of the white paper is to...
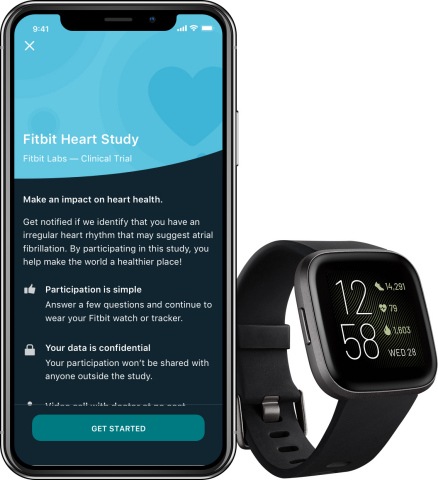
Fitbit has today launched the Fitbit Heart Study, its first large-scale, virtual study to validate the use of its wearable technology to identify episodes of irregular heart rhythm suggestive of atrial fibrillation (AF). This study is part of the...
The European Society of Cardiology (ESC) is for the first time in its 70-year history, to hold its annual meeting (ESC Congress) 100% online. A press release states that the move follows an announcement from the Dutch government banning...
The American College of Cardiology (ACC) and other North American cardiology societies, including the Canadian Association of Interventional Cardiology (CAIC), have issued guidance on ethically and safely reintroducing invasive cardiovascular procedures after the peak of COVID-19 has passed. The...
LifeSignals has received CE mark approval for the LifeSignals ECG Remote Monitoring Patch, a disposable wireless remote monitoring system, intended for use by healthcare professionals for continuous collection of Electrocardiography (ECG) and heart rate monitoring in ambulatory, hospital, healthcare...
Results of a clinical trial show stark disparities in the use of life-saving implantable cardioverter defibrillator (ICD) interventions based on sex and race, suggesting a potential bias in care pathways by electrophysiologists (EPs). The study was presented online as...
VitalConnect has been granted Emergency Use Authorisation (EUA) status by the US Food and Drug Administration (FDA) as part of the response to the COVID-19 pandemic. The EUA will enhance the capabilities of the VitalPatch and continuous patient monitoring...
A necklace that detects abnormal heart rhythm will be showcased for the first time on EHRA Essentials 4 You, a scientific platform of the European Society of Cardiology (ESC).
According to the study's author, Elmeri Santala from the University of...
A necklace that detects abnormal heart rhythm will be showcased for the first time on EHRA Essentials 4 You, a scientific platform of the European Society of Cardiology (ESC).
According to the study's author, Elmeri Santala from the University of...
A study published in the Journal of the American College of Cardiology (JACC) provides the first direct evidence that KCNQ1 antibodies are able to suppress arrhythmias in a cellular model of long QT syndrome type 2 (LQTS2), its authors...
Prism Schneider (Cumming School of Medicine, University of Calgary, Calgary, Canada) and others write in a commentary in the Canadian Medical Association Journal—because of an increase in domestic violence during the pandemic—healthcare providers should be aware of the signs...
Same-day discharge after atrial fibrillation (AF) ablation is feasible for the majority of patients when a standardised protocol is used, findings of a multicentre cohort study, published in the Journal of the American College of Cardiology (JACC), suggest. Efficacy and safety of a same-day discharge protocol were studied by...
On 18 March, the Centers for Medicare & Medicaid Services (CMS) recommended “limiting non-essential care and expanding surge capacity into ambulatory surgical centres and other areas” to conserve resources and staff for managing COVID-19 patients. However, in a statement...
iRhythm Technologies has announced a virtual platform to support patients and clinicians in the wake of COVID-19 by offering home enrolment for the company’s Zio XT and Zio AT heart monitoring devices.
Since patients with heart conditions are a particularly...
Coala Life has announced the expansion of capabilities to help monitor patients from home using the Coala Heart Monitor. This expansion is a result of the US Food and Drug Administration’s (FDA) new Emergency Guidance striving to help battle...
iRhythm Technologies has announced the appointment of Renee Budig to its Board of Directors effective 23 April 2020. Budig was appointed as a Class II director and will also serve on the Audit Committee.
“On behalf of the iRhythm team,...
The US Food and Drug Administration (FDA) has issued a Drug Safety Communication regarding known side effects of hydroxychloroquine and chloroquine, including serious and potentially life-threatening heart rhythm problems, that have been reported with their use for the treatment...
The use of mobile cardiac outpatient telemetry (MCOT) can help monitor COVID-19 patients taking hydroxychloroquine and azithromycin, while reducing potential exposure to the virus for clinicians and preserving personal protective equipment (PPE), according to a study published in the...
The Heart Rhythm Society (HRS) COVID-19 Rapid Response Task Force has updated its guidance on QTc monitoring in COVID-19 patients. In the revised guidance, the society provides recommendations for inpatient management of COVID patients recevieing hydroxychloroquine and advises against...
The European Society of Cardiology (ESC) is participating in two large-scale EU-funded projects to detect heart rhythm disorders and prevent sudden cardiac death.
The first of the projects, AFFECT-EU, aims to develop a universal screening algorithm for atrial fibrillation (AF),...
Cardiologs has announced the launch of a clinical study using the company’s artificial intelligence (AI) solution to remotely monitor the cardiac safety of COVID-19 patients during hydroxychloroquine treatment via analysis of electrocardiogram (ECG) data gathered from smartwatches.
Hydroxychloroquine therapy is...
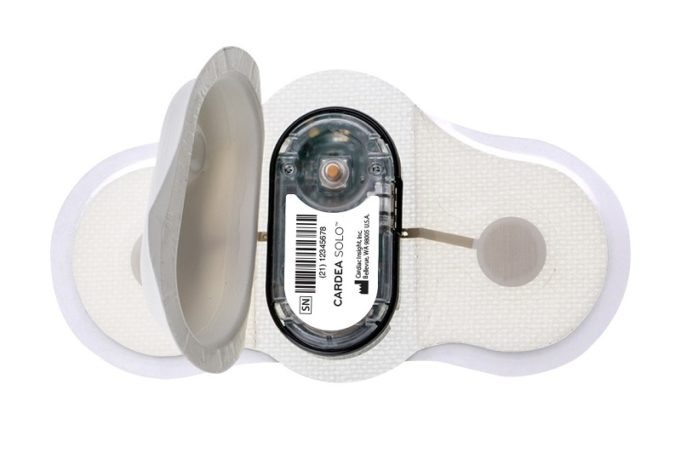
Cardiac Insight has announced the launch of the Heart@Home electrocardiogram (ECG) test kit to address new telehealth demands placed on cardiology practices by the COVID-19 response.
The kit enables physicians to prescribe the arrhythmia diagnosis technology of the Cardea SOLO...
In response to a recent American College of Cardiology (ACC) review that advocated that troponin should only be measured when a diagnosis of acute myocardial infarction is being considered, Andrew R Chapman (BHF Centre for Cardiovascular Science, Royal Infirmary...
DiNovA Medtech has announced that Vivek Reddy will join the company as chief medical advisor and co-founder of portfolio company Nuomao Medtech to lead cardiac arrhythmia related innovations.
Reddy is the Director of Cardiac Arrhythmia Services for the Mount Sinai...
The COVID-19 pandemic is hitting the world hard. Egypt, the most populous country in the Middle East and North Africa (MENA) region, is no exception. With more than 2,500 confirmed cases and a preliminary case fatality rate of 7%...
The American Heart Association (AHA) has compiled interim CPR guidelines to help rescuers treat cardiac arrest patients with suspected or confirmed COVID-19. The guidelines are in collaboration with the American Academy of Pediatrics, American Society of Anesthesiologists, American Association...
In a brief report in Annals of Internal Medicine, Seongman Bae (Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea) and colleagues report that neither surgical nor cotton masks appear to be an effective approach for preventing the dissemination of SARS-CoV-2 from...
Physicians already recognised the importance of remote management for heart failure patients long before the COVID-19 pandemic. However, the current situation has heightened the urgency of the transition for this vulnerable population. In this commentary, Michael Kiernan reviews how...
Bardy Diagnostics has announced that its Carnation Ambulatory Monitor (CAM) Patch has been chosen for use in protocols to measure QT Segments in COVID-19 patients who have been prescribed hydroxychloroquine (HCQ).
BardyDx’s CAM Patch will be utilised in newly established...
Presidents of leading cardiac organisations, including the American Heart Association (AHA), the American College of Cardiology (ACC) and the Heart Rhythm Society (HRS), have published a statement that cautions against the use of hydroxychloroquine and azithromycin for the treatment...
Royal Philips has announced that the US government and Philips agreed to team up to increase the production of hospital ventilators in its manufacturing sites in the USA.
Philips plans to double the production by May 2020 and achieve...
Medtronic has announced updates regarding its efforts to increase ventilator production around the globe. The company is announcing progress in the ramp-up of its ventilator production, as well as collaborating with technology partners and governments to drive new ventilator...
Medtronic has announced it is publicly sharing the design specifications for the Puritan Bennett 560 (PB 560) to enable participants across industries to evaluate options for rapid ventilator manufacturing to help doctors and patients dealing with COVID-19. This decision...
Cardiovascular specialists from across the globe shared current knowledge and key experience of managing COVID-19 patients as part of a webinar organised by the Imperial College Network of Excellence in Vascular Science at Imperial College London.
The four-part COVID-19 Cardiovascular...
The American Heart Association (AHA), in conjunction with the American Association for Respiratory Care (AARC) and American Society of Anesthesiologists (ASA) is offering guidance in oxygenation and ventilation management for healthcare providers as hospital volumes surge from COVID-19.
AHA released...
The reduced levels of angiotensin converting enzyme (ACE) 2 associated with cardiovascular disease and increasing age lead to a higher susceptibility to greater disease severity in COVID-19. A viewpoint published online in JAMA Cardiology hypothesises that severe acute respiratory...
The European Commission (EC) has adopted a proposal to postpone by one year the date of application of the new Medical Devices Regulation (MDR), which was due to come into force on 26 May this year. The postponement, a press...
The Heart Failure Association (HFA) of the European Society of Cardiology (ESC) has announced that Heart Failure 2020 & the World Congress on Acute Heart Failure, scheduled to take place over 23–26 May in Barcelona, Spain, has been cancelled.
In...
Athena Poppas (Providence, USA) is the new president of the American College of Cardiology (ACC). Her term officially began at the close of the ACC/World Congress of Cardiology’s virtual scientific sessions (ACC.20/WCC Virtual).
Poppas has been a long-standing leader of...
Simon Ray, president of the British Cardiovascular Society (BCS), has told the COVID-19 Cardiovascular Conference that a lack of consistency in national guidance around personal protective equipment (PPE) during COVID-19 had led to a “vacuum” which has had to...
In a new statement published on 2 April 2020, all 45 societies represented by the US Council of Medical Specialty Societies (CMSS)—over 800,000 physicians—emphatically declare their belief that all frontline healthcare professionals must have access to personal protective equipment...
A raised troponin level in myocardial injury “greatly alters the prognosis adversely” in COVID-19 patients, Graham Cole (Imperial College Healthcare NHS Trust, London, UK) told a virtual cardiovascular conference, hosted today by Imperial College London.
Cole explained: “Troponin rises occur...
The COVID-19 Cardiovascular Conference, hosted as a live webinar by The Imperial College Network of Excellence in Vascular Science at Imperial College (London, UK), has heard about experiences from both Italy and the USA, as well as details of...
The Heart Rhythm Society (HRS), The American College of Cardiology (ACC), and the American Heart Association (AHA) have released a joint document dealing with the impact of COVID-19 on electrophysiology.
Published online in Heart Rhythm by Dhanunjaya Lakkireddy (Kansas City...
Due to COVID-19 concerns and travel restrictions, the 2020 Heart Rhythm Scientific Sessions (HRS 2020) will not take place in San Diego, USA, on 6–9 May as planned, the Heart Rhythm Society has announced.
Instead, HRS will offer several new...
Daniel M Philbin Jr has been named chair of the American College of Cardiology (ACC) board of governors and secretary of the board of trustees, the main governing body of the ACC, for 2020–2021. His term began at the...
The Imperial College Network of Excellence in Vascular Science at Imperial College London (UK) is to run a COVID-19 Cardiovascular Conference as a live webinar on Thursday 2 April for all healthcare professionals battling COVID-19.
The webinar aims to share...
Results from the phase 2 MAVERICK-HCM clinical trial, presented at the American College of Cardiology/World Congress of Cardiology’s virtual scientific sessions (ACC.20/WCC Virtual) suggest that the use of mavacamten (MyoKardia) for the treatment of non-obstructive hypertrophic cardiomyopathy (nHCM) may be...
Data on the use of LivaNova’s implantable neuromodulation technology, Vitaria, for heart failure patients has been shared at the American College of Cardiology/World Congress of Cardiology’s virtual scientific sessions (ACC.20/WCC Virtual).
Two posters were published and shared on demand at the...
The infusion of ethanol via the vein of Marshall (VOM), coupled with catheter ablation, leads to improved outcomes for patients with persistent atrial ablation, according to the findings of a study presented by Miguel Valderrabano (Baylor College of Medicine, Houston, USA) at the American College of Cardiology/World Congress of Cardiology’s...
Data from the National Cardiovascular Data Registry (NCDR) Left Atrial Appendage Occlusion (LAAO) Registry, which enrolled more than 38,000 patients implanted percutaneously with the Watchman device (Boston Scientific), reveal a high implant success rate and a low rate of...
During a Q&A section of a European Commission (EC) college meeting on 25 March, EC spokesperson Stefan de Keersmaecker stated that the commission were looking to delay the “entry into force” of the new European medical device regulations (MDR) because of...
A viewpoint in the Journal of the American Medical Association (JAMA) has offered potential solutions to modifying ongoing randomised clinical trials during the COVID-19 pandemic. It aims to “minimise disruption and preserve integrity”, while still ensuring participant health and...
Cardiac injury is common among hospitalised patients with COVID-19, and is associated with a higher risk of in-hospital mortality, a study from Wuhan, China, has shown. Although the exact mechanism of cardiac injury has not been defined, the researchers...
The US Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) has written to the medical device industry to outline its response to the COVID-19 public health emergency in its day-to-day operations with industry.
The letter from...
The American Heart Association (AHA) has committed US$2.5 million to research efforts to better understand the novel coronavirus, COVID-19, and its interaction with the body’s cardiovascular and cerebrovascular systems.
Specifically, the Association will be offering fast-tracked research grants for short-term...
The British Cardiovascular Intervention Society (BCIS) and the British Cardiovascular Society (BCS) have endorsed guidance published by NHS England and NHS Improvement on the provision of cardiology services during the COVID-19 pandemic.
A joint statement from Nick Curzen, president of...
Aktiia, a Swiss-US start-up that has developed an optical blood pressure monitor at the wrist, has raised an additional CHF 6 million in funding to bring its product to market. The technology combines common optical sensors and proprietary clinically...
AliveCor has announced that its KardiaMobile 6L is now allowed for use in the measurement of a patient’s QTc and detection of potentially dangerous QT prolongation. A prolonged QTc can lead to a potentially fatal side effect of drug-induced...
A case series of US experience with COVID-19 has upheld suggestions of a link with cardiomyopathy, which developed in 33% of intensive care patients.
In a research letter published in the Journal of the American Medical Association, Matt Arentz (Department...
The US Food and Drug Administration (FDA) has issued an immediately-in-effect guidance that allows manufacturers of certain FDA-cleared non-invasive, vital-sign-measuring devices to expand their use so that health care providers can use them to monitor patients remotely.
The devices...
The American College of Cardiology Scientific Session Together with World Congress of Cardiology (ACC.20/WCC) is to run as a virtual meeting, following its cancellation due to the coronavirus outbreak. It was scheduled to take place on 28–30 March 2020...
Portola Pharmaceuticals announced new data that they say reinforces the value of Andexxa (coagulation factor Xa , inactivated-zhzo). The company statement outlines that the data demonstrate Andexxa was associated with a lower rate of in-hospital and 30-day mortality in...
Cephalic vein cutdown (CVC) and axillary vein access (AVA) are both effective approaches for cardiac implantable electronic device (CIED) lead implantation, and could avoid the complications usually observed with traditional subclavian vein puncture (SVP). This was the conclusion of...
Researchers have derived several algorithms to identify emergency department (ED) oral anticoagulant prescriptions for patients with atrial fibrillation (AF) in a large health dataset. The retrospective cohort study identified algorithms that, they say, “can be selected to optimise specificity,...
Almost 70% of patients treated with a leadless left ventricular (LV) endocardial pacing system had a favourable clinical response at six months, a post-market surveillance registry has shown.
The findings were published in Heart Rhythm. Authors Benjamin J Sieniewicz (St...
Miguel Angel Cobos Gil (Instituto Cardiovascular, Hospital Clinico San Carlos, Madrid, Spain) outlines in the Annals of Internal Medicine a novel method of using the Apple Watch to record a multi-lead, quasi-standard electrocardiogram (ECG). He reports that the method...
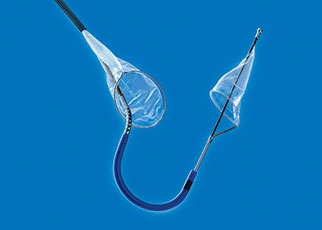
The novel left atrial appendage (LAA) closure device Appligator (Append Medical) was a finalist in the 2019 ICI Innovation Award Competition (Innovation in Cardiovascular Interventions 2019; 8–10 December, Tel Aviv, Israel). Leonid Sternik, who came up with the concept...
Impulse Dynamics, developer of cardiac contractility modulation (CCM) therapy delivered by the Optimizer Smart System, has announced the appointment of Ishu Rao as medical director.
A press release from the company states that Rao is a board-certified cardiac electrophysiologist who...
The scientific congress of the European Heart Rhythm Association (EHRA 2020) and the Scientific Session of the American College of Cardiology Together with World Congress of Cardiology (ACC.20/WCC) have both been cancelled due to the coronavirus outbreak.
EHRA 2020 was...
Stereotaxis has received US Food and Drug Administration (FDA) 510(k) clearance for the Genesis Robotic Magnetic Navigation (RMN) system for the robotic navigation of magnetic ablation catheters to treat heart rhythm disorders.
“Genesis is a leap forward in Robotic Magnetic...
James Teo was the chief investigator in the EPACS clinical trial, testing the performance of the Zio Patch (iRhythm Technologies) wearable biosensor. He outlines to Cardiac Rhythm News his experience with the device and discusses its use in the...
Rapid high-current, temperature-controlled point-by-point pulmonary vein isolation (PVI) and linear ablation has been shown to be clinically feasible and safe in a first-in-human study of an expandable radiofrequency lattice tip catheter. Other findings from the same study, announced last...
Twenty late-breaking trials, including major clinical studies on the treatment of atrial fibrillation (AF) are among the research to be presented at the annual congress of the European Heart Rhythm Association (EHRA) 2020 (29–31 March, Vienna, Austria).
The late-breaking trials...
Kalyanam Shivkumar has been named the next editor-in-chief of the Journal of the American College of Cardiology (JACC): Clinical Electrophysiology.
Shivkumar is a physician scientist who serves as the director of the University of California, Los Angeles (UCLA) Cardiac Arrhythmia...
Wearing an adhesive patch sensor that streams data to an analytical platform can accurately predict worsening heart failure (HF) and the need for hospitalisation several days before it is necessary, according to research published in Circulation: Heart Failure.
The LINK-HF...
Append Medical, developer of the Appligator, a novel left atrial appendage (LAA) closure device to minimise stroke risk in atrial fibrillation (AF) patients, and Sheba Medical Center (Ramat Gan, Israel), have announced the clinical proof-of-concept data evaluating the safety...
Abbott has announced that it has received CE mark approval for the Gallant implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) devices.
The devices offer new opportunities for patient engagement and remote monitoring through new smartphone connectivity and...
A study of patients undergoing implantable cardioverter defibrillator (ICD) therapy across three centres, found that only one in three were treated in concordance with current guidelines on ICD device programming. This is the finding of research led by Teetouch...
VitalConnect has announced the introduction of arrhythmia detection to its remote monitoring portfolio. In partnership with CorVitals, VitalConnect now offers physicians the ability to monitor, diagnose and treat patients suffering from various heart conditions and diseases.
With arrhythmia detection, clinicians...
The American College of Cardiology (ACC) and automated external defibrillator (AED) technology start-up HeartHero have formed an alliance aimed at making a “significant impact” on survival rates after sudden cardiac arrest (SCA).
The partnership will further ACC’s mission to transform...
The US Food and Drug Administration (FDA) has authorised marketing of software to assist in the acquisition of cardiac ultrasound, or echocardiography, images. The software, called Caption Guidance (Caption Health), is an accessory to compatible diagnostic ultrasound systems and...
CardioFocus has submitted a pre-market approval (PMA) supplement to the US Food and Drug Administration (FDA) for the HeartLight X3 Endoscopic Ablation System for the treatment of atrial fibrillation (AF). This supplement follows the original, approved PMA of the...
Jennifer Silva (St Louis, USA) talks to BLearning Cardio at SIR 2019 (Society of Interventional Radiology; 23–28 March; Austin, USA) about why physicians should move away from using Virtual Reality (VR) during procedures and move towards augmented and mixed...
RHYTHM AI has announced the signing of a research collaboration agreement with Biosense Webster. A press release from RHYTHM AI outlines that the agreement grants it access to certain data from the Biosense Webster CARTO 3 System to support...
Abbott has announced approval from the US Food and Drug Administration (FDA) for the CATALYST trial to examine its Amplatzer Amulet device compared to non-vitamin K oral anticoagulants to lower stroke and bleeding risks for patients with atrial fibrillation.
A...
Medtronic has received CE Mark for its Cobalt and Crome portfolio of implantable cardioverter-defibrillators (ICDs) and cardiac resynchronisation therapy-defibrillators (CRT-D). ICDs monitor heart rhythms and deliver therapy to correct heart rates that are too fast and can lead to...
Eko has announced that the US Food and Drug Administration (FDA) has cleared a suite of algorithms that, when combined with Eko’s digital stethoscopes, will enable healthcare providers to more accurately screen for heart conditions during routine physical exams....
Hugh Calkins (Johns Hopkins Medical Institutions, Baltimore, USA) told delegates attending the AF Symposium 2020 (23–25 January, Washington, DC, USA) that cryoablation with the Arctic Front catheter (Medtronic) was a safe and effective approach for managing persistent atrial fibrillation...
At the AF Symposium 2020 (23–25 January, Washington, DC, USA), Vivek Y Reddy (Helmsley Electrophysiology Center, Icahn School of Medicine at Mount Sinai, New York, USA) reported that pulsed field ablation (PFA)—with the Farawave (Farapulse Inc.) catheter—may be a feasible and...
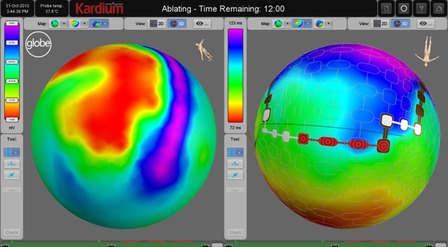
Acutus Medical offered a demonstration of its AcQMap advanced cardiac imaging and mapping system at AF Symposium (23–25 January, Washington, DC, USA) with a live-stream of a case study from Oxford University Hospitals. The live stream, which was followed by...
Speaking today at the AF Symposium (23–25 January, Washington, DC, USA), Francis Marchlinski (Hospital of the University of Pennsylvania, Philadelphia, USA) reported that selected atrial fibrillation (AF) patients could safely and effectively take non-vitamin K antagonist oral anticoagulants (NOACs)...
Medtronic has received approval from the US Food and Drug Administration (FDA) to proceed with an investigational device exemption (IDE) trial to evaluate the safety and effectiveness of the PulseSelect Pulsed Field Ablation (PFA) System, a new technology that...
Bardy Diagnostics (BardyDx) has today announced the commercial launch of the 14-Day version of the Carnation Ambulatory Monitor (CAM), the P-wave centric ambulatory cardiac patch monitor and arrhythmia detection device, following recent clearance by the FDA.
“The 14-Day CAM...
Biotronik has announced the market release of its injectable cardiac monitor (ICM), BioMonitor III in Japan. The device is designed to help patients with irregular heart rhythms by documenting unexplained syncope with increased clarity.
In a press release, Biotronik noted...
A technique that enables patients suffering from heart conditions to hold their breath safely for over five minutes could have potential as a treatment for cardiac arrhythmias, according to research lead by Michael Parkes (University of, and University Hospitals...
Malcom Finlay (Bart's Health Centre, London, UK) reviews the growing role of artificial intelligence (AI) in diagnosing arrhythmias.
Johnny, a 72-year-old film producer, is meeting clients when his watch gently vibrates notifying him of his irregular pulse. He pressed his...
Medtronic has announced that it has received US Food and Drug Administration (FDA) approval of Micra AV, the world’s smallest pacemaker with atrioventricular (AV) synchrony.
Micra AV is indicated for the treatment of patients with AV block, a condition in...
CardioFocus, has announced that the company’s board of directors has promoted Burke T Barrett to the position of chief executive officer (CEO), effective January 19, 2020. Barrett previously served as CardioFocus’ president and chief operating officer (COO).
The company is...
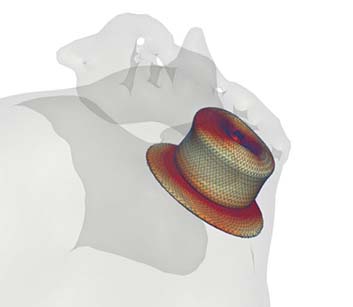
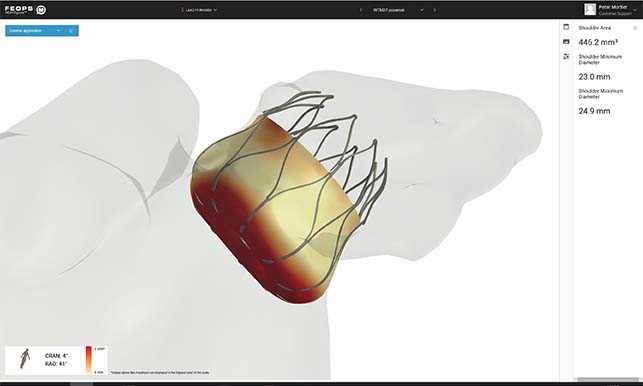
FEops has announced that the first patient has been enrolled in the physician-initiated PREDICT-LAA trial.
The trial is led by Righshospitalet (Copenhagen, Denmark) and aims to assess whether the use of FEops HEARTguide computer simulations based on cardiac CTimaging can...
Highlights:
WRAP-IT shows significant mortality risk with CIED infection, and reduced quality of life
Sequential approach using STAR mapping system identifies key early sites of activation in patients with persistent AF
Profile: Eric N Prystowsky
Andrew Grace discusses how...
Leading electrophysiologists have pinpointed four clinical studies due to report findings in 2020—AMULET IDE, aMAZE IDE, PULSED AF and CEASE AF—that they expect to shape practice in the treatment of cardiac arrhythmias this year.
Dhanunjaya Lakkireddy (chair of the American...
Andrew Grace charts the evolution of non-contact mapping culminating in the current AcQMap system—the technology has advanced understanding of persistent atrial fibrillation (AF), providing further support for the idea that AF is, in fact, a disorder of cardiac development.
Electromagnetic...
Eric N Prystowsky (director of the Cardiac Arrhythmia Service at St Vincent Hospital, Indianapolis, and a consulting professor of medicine at Duke University Medical Center, Durham, USA) talks to Cardiac Rhythm News about his distinguished career. He considers the...
The UK National Institute for Health and Care Excellence (NICE) has issued draft guidance recommending the use of the Reveal LINQ (Medtronic) implantable cardiac monitor for the diagnosis of atrial fibrillation (AF).
The draft guidance recommends the routine adoption in...
Bardy Diagnostics (BardyDx) has announced today the appointment of Ed Vertatschitsch as chief operating officer. Effective 2 January 2020, the appointment will see Vertatschitsch responsible for leading all operations, research & development, and regulatory and quality affairs functions, the...
The American College of Cardiology (ACC) has announced several promotions and structural changes within its senior staff. The College said that the staff changes will “further consolidate and strengthen the organisation’s efforts in achieving its strategic goals, mission and...
Stryker has launched a voluntary field action on specific units of the LIFEPAK 15 monitor and defibrillator.
In an announcement issued by the company on 27 December 2019, and subsequently via the US Food and Drug Administration (FDA) on 10...
AtriCure has announced the successful completion of patient enrolment in the aMAZE clinical trial. In addition, the company announced that it has received approval from the US Food and Drug Administration (FDA) for the continuation of the trial under...
Abbott has announced US Food and Drug Administration (FDA) approval of a new alternative surgical technique for Abbott's HeartMate 3 heart pump that the company says will allow more advanced heart failure patients to avoid open heart surgery. The...
The US Food and Drug Administration has granted Breakthrough Device designation to the digital health and artificial intelligence (AI) company Eko for an echocardiogram (ECG)-based algorithm to help identify induced Left Ventricular Ejection Fraction (LVEF).
FDA Breakthrough Device designation helps...
Daiichi Sankyo Europe has announced outcomes from an observational study in mainly caucasian atrial fibrillation (AF) patients being treated with the anti-coagulation drug edoxaban (Lixiana).
The results of the Danish observational cohort study, published in the European Heart Journal...
Stereotaxis announced today that HCA Midwest Health has launched a new robotic arrhythmia care programme based at Overland Park Regional Medical Center (OPRMC). Installation of the Stereotaxis Robotic Magnetic Navigation (RMN) System was recently completed, and the first patients...
The US Food and Drug Administration (FDA) Cardiovascular and Renal Drugs Advisory Committee (CRDAC) has voted not to recommend approval for the anti-arrhythmic drug Brinavess (vernakalant hydrochloride, IV; Correvio). The CRDAC met last week (10 December) to review Correvio’s...
Data from the CAP and CAP2 registries—which contain the longest and largest follow-up of patients—add to previous evidence that left atrial appendage closure (LAAC) with Watchman (Boston Scientific) is a safe and effective alternative to long-term anticoagulation in patients...
Impulse Dynamics has announced the completion of a US$80.25 million Series D financing round with new investors. Proceeds will be used primarily to facilitate US commercialisation of the Optimizer Smart, an FDA-approved cardiac contractility modulation (CCM) therapy delivery device....
Uncovering and abolishing focal ventricular tachycardia (VT) may further improve outcomes of catheter ablation in the treatment of structural heart disease, a study published in the Journal of the American College of Cardiology (JACC): Clinical Electrophysiology concludes. Robert Anderson...
VivaLnk has announced it has received the class IIa medical device CE mark for its multi-vital medical wearable sensor and software development kit (SDK).
The multi-vital wearable sensor can generate a continuous stream of ECG rhythm, respiratory rate, heart rate,...
Five independent predictors of cardiac implantable electronic device (CIED) infection have been identified which could be used to aid physicians and patients in decision-making about device therapy through the use of an infection risk scoring system. The predictors and...
With his brothers Josep and Ramon, Pedro Brugada discovered the syndrome that bears their name. He reflects on his career highlights with Cardiac Rhythm News, charting the development of electrophysiology from esoteric to mainstream therapy, and stresses the role...
Using artificial intelligence (AI) to examine echocardiograms (ECGs) can identify patients at risk of developing atrial fibrillation (AF), as well as accurately predict one-year all-cause mortality, data presented at the American Heart Association Scientific Sessions (AHA 2019; 16-18 November,...
Major infection in patients with cardiac implantable electronic devices (CIED) is associated with a threefold risk of mortality at one year, and impaired quality of life through six months, according to data presented by Bruce Wilkoff (Cleveland Clinic, Cleveland,...
Biosense Webster, a Division of Johnson & Johnson, has reaffirmed its commitment to tackling atrial fibrillation (AF) by launching two major initiatives to coincide with Global AF Aware Week, 18–24 November 2019. A press release from the company outlines...
Children and teens with cardiac arrhythmias are more likely to have depression, anxiety and attention deficit hyperactivity disorder (ADHD) compared with those of similar ages without chronic medical conditions or with certain select chronic childhood diseases, according to preliminary...
Correvio announced the presentation of new data from the SPECTRUM study evaluating Brinavess (vernakalant hydrochloride, IV), an antiarrhythmic drug for the rapid conversion of recent onset atrial fibrillation (AF), at the American Heart Association (AHA) 2019 Annual Meeting (16–18...
Final results from the Apple Heart study demonstrate a low probability of receiving an irregular pulse notification. It also found that 34% of those who received notification of an irregular pulse were diagnosed with atrial fibrillation (AF) on subsequent...
Monday 18 November marks the start of Global AF Aware Week 2019, launched by the AF Association. The annual awareness week is dedicated to raising awareness of atrial fibrillation (AF), through the association’s Detect, Protect, Correct, Perfect campaign.
A press...
A joint press release from Bristol Myers Squibb and Pfizer has highlighted the findings of an artificial intelligence (AI)-based machine learning (ML) technique that has been shown in a test database to exhibit greater predictive performance than other currently...
A wrist-based optical blood pressure monitor has the potential to replace cuff monitoring systems for ambulatory blood pressure, suggests a study in the journal Blood Pressure Monitoring. A press release from Aktiia, the Swiss-based company manufacturing the device, states...
Medtronic has announced results from the MARVEL 2 (Micra Atrial Tracking Using A Ventricular accELerometer) study. A press release from the company says that the study shows that an investigational set of algorithms in the Micra Transcatheter Pacing System...
Highlights:
ESC guidelines on supraventricular tachycardias underline the pivotal role of catheter ablation therapy
Study suggests increased use of DOACs in AF leads to lower healthcare costs
Profile: Pedro Brugada
Cynthia James discusses how she and colleagues developed a...
Left atrial appendage closure (LAAC) via implanted device was associated with a decreased risk of admission for stroke, compared with the expected risk without anticoagulation therapy, according to a study published in the Journal of the American Medical Association...
Stereotaxis and ADAS 3D Medical have announced that the first patients have been treated with the integration of ADAS 3D’s advanced preoperative substrate mapping and Stereotaxis’ robotic magnetic navigation technologies.
ADAS 3D helps identify possible sources of dangerous irregular heartbeats...
CardioFocus has announced that the first patients in France have been treated in the University Public Hospital of Nancy with the HeartLight endoscopic ablation system as part of a distribution partnership with MicroPort CRM France.
To date, more than 7,000...
Cardiva Medical has said that results from the AMBULATE pivotal study demonstrate the safety and efficacy of its VASCADE MVP venous vascular closure system compared to manual compression. The results were published online in the Journal of the American...
Early mortality following atrial fibrillation (AF) ablation affects nearly one in 200 patients, with the majority of deaths occurring during 30-day readmission, an analysis of a nationally representative US cohort has revealed. Writing in the Journal of the American...
Bardy Diagnostics announced that it was selected as winner of the ‘Remote Monitoring in Arrhythmias’ Technology and Innovation Pitch Session held as part of the digital health programme at the European Society of Cardiology (ESC) Congress 2019 (31 August–4...
The use of defibrillation testing (DFT) when inserting an implantable cardioverter defibrillator (ICD) declined significantly in US hospitals between 2010 and 2015; during the same time period there was an increase in institutional variability in performing DFT that was...
Different strategies are needed to address the continuing lack of diversity in the US cardiology workforce, say the authors of a cross-sectional study which found that numbers of underrepresented minority (URM) individuals and women remain proportionately lower, and that...
Daiichi Sankyo has announced one-year follow-up results from an analysis of 12,574 European non-valvular atrial fibrillation (NVAF) patients, mostly elderly, treated with edoxaban (Lixiana). The results from the ongoing Global ETNA-AF registry, were presented at the Great Wall International...
iRhythm Technologies has launched its Zio service in the UK to support the identification and clinical diagnoses of cardiac arrhythmias such as atrial fibrillation (AF).
To aid in the diagnosis of the condition, iRhythm has developed Zio, a wearable...
The US Food and Drug Administration (FDA) has approved Farxiga (dapaglifozin, AstraZeneca) to reduce the risk of hospitalisation for heart failure in adults with type 2 diabetes and established cardiovascular disease (CVD) or multiple cardiovascular (CV) risk factors. The...
Acutus Medical and Innovative Health have announced a partnership in electrophysiology that they say will offer advanced technology to improve patient outcomes in a cost-efficient manner. A joint press release from the two venture-backed companies says that they will...
Douglas Drachman has been selected as the next vice chair of the American College of Cardiology’s Annual Scientific Session. Drachman will serve as vice chair for two years, at ACC ‘21 and ACC ‘22, and then as chair for...
Researchers have used cardiac magnetic resonance (CMR) imaging for the first time to demonstrate the mechanistic improvements in cardiac contractility consequent to cardiac resynchronisation therapy (CRT), as well as the relative safety of the approach. Presenting the findings this...
Osita Okafor (Aston University, Birmingham, UK) presented an oral abstract this week at the Heart Rhythm Congress (HRC 2019; 6–9 October, Birmingham, UK) that demonstrated that post-implantation changes in QRS area, derived from vectorcardiography (VCG), are superior to QRS...
The use of a Stochastic Trajectory Analysis of Ranked signals (STAR) mapping approach consistently identified early sites of activation (ESA) in patients with persistent atrial fibrillation (AF), and the ablation response was compatible with these ESA being driver sites....
Hugh Calkins (Baltimore, USA) tells BLearning Cardio at Venice Arrhythmias 2019 (3–5 October; Venice, Italy) that the 2019 HRS consensus document is extremely important because it defines a new disease and that this broad group of patients need careful evaluation and detailed management.
Electrophysiologists must read...
Antonio Raviele (Venice, Italy), the president of Venice Arrhythmias 2019 (3–5 October; Venice, Italy), talks to BLearning Cardio about some of the current challenges in atrial fibrillation (AF) management, which he notes include lifestyle modification and risk factor modification and the risk to...
Orchestra BioMed, a biomedical innovation company, announced results from its MODERATO II double-blind, randomised study of BackBeat Cardiac Neuromodulation Therapy (CNT). In a press release, the company states that it demonstrated statistically significant and clinically meaningful reductions in systolic...
Data from an interim analysis of heart failure patients treated in the CorCinch FMR study show, a press release reports, that the AccuCinch ventricular repair system (Ancora Heart) is associated with a favourable safety profile, with 97% freedom from...
A prespecified analysis of data from the AUGUSTUS study, released at late-breaking session at the Transcatheter Cardiovascular Therapeutics scientific symposium (TCT 2019; 25–29 September, San Francisco, USA) and published online in Circulation, indicate that anticoagulation with apixaban and a...
A new study from New Zealand, just published in the New Zealand Medical Journal, has revealed that automated external defibrillators (AEDs) in the community are, alarmingly, not “actually that accessible”. The study found there were only three devices in Hamilton that...
Primary prevention of arrhythmic events in arrhythmogenic right ventricular cardiomyopathy (ARVC) remains challenging; Cynthia James explains how she and colleagues developed a model for selecting patients suitable for placement of implantable cardioverter-defibrillators (ICDs), and what further refinements are needed.
Arrhythmogenic...
Disclaimer: This advertorial is sponsored by Abbott
The Advisor™ HD Grid Mapping Catheter, Sensor Enabled™ (SE), is a unique mapping catheter that, when used with the EnSite Precision™ Cardiac Mapping System, allows bipole recording of waveforms in both the parallel...
Disclaimer: Advertorial sponsored by Abbott
Launched in May 2019 by Abbott, the next-generation Confirm Rx™ insertable cardiac monitor (ICM) has both the CE Mark in Europe and Food and Drug Administration clearance in the United States. The Confirm Rx ICM...
The US Food and Drug Administration (FDA) has granted Fast Track designation for the development of dapagliflozin (Farixga, AstraZeneca) to reduce the risk of cardiovascular death or the worsening of heart failure in adults with heart failure with reduced...
Stereotaxis has appointed Kimberly Peery as chief financial officer (CFO), effective from 1 October 2019. Martin Stammer, who has served as CFO since 2013, has accepted a position as the CFO of a large professional services firm headquartered in...
EBR Systems has announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its WiSE cardiac resynchronisation therapy (CRT) for the treatment of heart failure. The WiSE (wireless stimulation endocardially) CRT system is designed...
Jenny Bjerre outlines the results of a study presented at the European Society of Cardiology Congress (ESC 2019; 31 August–4 September, Paris, France) which examined the information on driving restrictions provided to patients following implantable cardioverter defibrillator (ICD) therapy,...
Personalised education delivered in a home setting significantly reduces hospitalisations in patients with atrial fibrillation (AF). This was among the findings of the HELP-AF (Home-based education and learning programme) study, which was presented by Prashanthan Sanders (University of Adelaide...
A study that compared the use of maximum-fixed energy and low-escalating energy shocks for cardioversion in patients with atrial fibrillation (AF) found that maximum-fixed shocks were significantly more effective at cardioverting AF. No differences were found in any safety...
AtriCure has entered into a definitive agreement to acquire SentreHEART, which developed the LARIAT device for left atrial appendage closure in patients with atrial fibrillation. A press release reports that the transaction consideration consists of an upfront payment of...
Virtual patient care is creating exciting learning opportunities for interventional cardiologists. Atman P. Shah considers how training through gaming can deliver a state-of-the-art profession.
Recently, I was between patients, with a few minutes to spare. I opened an app on...
RHYTHM AI has announced the closure of a £2.15 million seed financing round. The round was led by an affiliate of Rinkelberg Capital, a private wealth management firm based in London, and was supported by investment from founders. The...
iRhythm Technologies has announced a collaboration with Verily to develop solutions aimed at improving the screening, diagnosis, and management of patients with atrial fibrillation (AF).
A press release from iRhythm says that the collaboration brings together its expertise in...
Omar Ishrak, Medtronic’s chairman and CEO is to retire on 26 April 2020, following the end of the company’s current fiscal year. Also, the Medtronic Board of Directors has announced key leadership appointments as part of its multi-year, leadership...
New data from the Swedish Heart Failure Registry indicate the use of an implantable cardioverter defibrillator (ICD) in patients with heart failure with reduced ejection fraction (HFrEF) is associated with a significant reduction in all-cause mortality at both one...
Results from the Atrial fibrillation progression trial (ATTEST) were presented on 31 August at the European Society of Cardiology (ESC) Congress 2019 in Paris, France. ATTEST is the first randomised controlled trial to directly compare the effectiveness of ablation...
At the 2019 European Society of Cardiology (ESC) Congress (31 August–4 September, Paris, France), Pavel Osmancik (Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic) presented the results of PRAGUE-17. These showed that left atrial appendage closure (LAAC)...
A four-year Italian study of oral anticoagulant (OAC) use in patients with atrial fibrillation (AF) has shown that as prescription rates increased healthcare costs per patient decreased. Prescription rates for antiplatelet agents fell during the same time period. Other...
The European Society of Cardiology (ESC) unveiled guidelines for treatment of supraventricular tachycardia (SVT) on the opening day of its Congress (ESC 2019; 31 August–4 September, Paris, France). Simultaneously published online in the European Heart Journal, the document provides...
A retrospective analysis of data from the Framingham Heart Study has determined that, although heavy smokers have a significantly decreased risk of cardiovascular disease (CVD) within five years after smoking cessation in comparison to current smokers, their risk remains...
Applying artificial intelligence (AI) to a standard electrocardiogram (ECG) may be used to determine physiological age, according to a study published in Circulation: Arrhythmia and Electrophysiology. Zachi Attia (Mayo Clinic, Rochester, USA) et al found that an AI algorithm...
William W Pinsky and Mandeep R Mehra explain to Cardiovascular News how international medical graduates contribute to both their adopted country and their country of origin, providing benefits for all.
It should come as no surprise that international medical graduates...
Philips is showcasing its latest cardiac care innovations at the ESC Congress 2019 (European Society of Cardiology; 31 August–4 September, Paris, France). At the congress, Philips is showcasing Release 5.0 of its EPIQ CVx cardiology platform for the first...
AtriCure has received US Food and Drug Administration (FDA) 510(k) clearance of additional labelling claims for AtriClip LAA management devices, including changing the indication from occlusion of the LAA to exclusion, and also adding electrical isolation as a labelling...
ZOLL Medical has confirmed that it has completed the previously announced acquisition of Cardiac Science Corporation, a leading provider of automated external defibrillators (AEDs), related services, and accessories.
"We are excited about the alignment of our two companies and our combined ability...
The first patient has been enrolled in the CorCinch EU study, which is a European multicentre clinical evaluation of the AccuCinch ventricular repair system (Ancora Heart) as a treatment for patients with reduced ejection fraction systolic heart failure. This...
AtriCure has revealed that it has entered into a definitive agreement to acquire SentreHeart. Under the terms of the acquisition agreement, the transaction consideration consists of an upfront payment of approximately US$40 million in cash and AtriCure common stock,...
New results support the feasibility of an entirely extracardiac pacing method in a heterogeneous patient population, using a minimally invasive, parasternal delivery approach, with adequate sensing and thresholds suited for temporary pacing. The study, authored by Anne-Floor BE Quast,...
By Prof Dr Christoph T Starck, FEHRA This advertorial was sponsored by Cook Medical
We report the case of a 74-year old-male patient with a cutaneous device perforation due...
Biotronik has announced the publication of the SENSE trial results in the Journal of Cardiovascular Electrophysiology, proving Biotronik's DX system is equivalent to dual-chamber implantable cardioverter defibrillators (ICDs) in detecting atrial high-rate episodes (AHREs). Trial data also demonstrate that...
SentreHeart recently announced it has successfully completed nine first-in-human procedures utilising the Lariat with EpiRail at John Paul II hospital in Krakow, Poland. The Lariat with EpiRail is an epicardial-only, percutaneous approach to complete and permanent closure of the...
An artificial intelligence (AI) model has been found to identify patients with intermittent atrial fibrillation even when performed during normal rhythm using a quick and non-invasive 10 second test, compared to current tests which can take weeks to years....
A study of 174 hospitals in Australia and New Zealand, published recently in the Annals of Internal Medicine, shows that the quality of care people receive may account for the wide variation in the rate of complications after having...
Roderick Tung (Chicago, USA) talks to BLearning Cardio about the results of the His-SYNC trial which he presented at HRS 2019.
The His-SYNC trial set out to compare the effectiveness of His bundle pacing compared with biventricular pacing as first-line therapy in...
Acutus Medical has announced the publication of the UNCOVER AF study in Circulation: Arrhythmia and Electrophysiology. The study demonstrated 73% single-procedure freedom from atrial fibrillation (AF) at 12 months with the use of Acutus' AcQMap advanced cardiac imaging and...
In a large, real-world population of cardiac resynchronisation therapy recipients followed by remote monitoring, use of the adaptive cardiac resynchronisation therapy biventricular and left ventricular algorithm compared to standard biventricular pacing was associated with a “significantly reduced risk” of...
The American College of Cardiology/American Heart Association (ACC/AHA) recently published new primary prevention of heart disease guidelines. The guidelines focus on traditional recommendations for managing conditions like atrial fibrillation (AFib), irregular heartbeat, and atherosclerosis that put someone at increased...
Biotronik has announced FDA clearance of the BioMonitor III injectable cardiac monitor (ICM). BioMonitor III is designed to document suspected arrhythmia or unexplained syncope with increased clarity, enabling fast diagnosis and appropriate treatment. This news follows the recent European...
A new method of evaluating irregular heartbeats outperformed the approach that is currently used widely in stroke units to detect instances of atrial fibrillation.
The technology, called electrocardiomatrix, goes further than standard cardiac telemetry by examining large amounts of telemetry...
Medtronic has announced plans for a global registry of its HeartWare HVAD System, following the release of two-year outcomes from the LATERAL clinical trial. The HVAD System is a left ventricular assist device (LVAD) that helps increase the amount...
A pooled analysis of five-year data from the PROTECT AF and PREVAIL trials has shown left atrial appendage closure (LAAC) to be cost‐effective, and to save costs in comparison to warfarin or non-vitamin K antagonist oral anticoagulant (NOAC) therapy.
Published...
Christoph Starck (Berlin, Germany) and Saumya Sharma (Houston, USA) discuss the benefits of using rotational transvenous lead extraction (TLE) tools. Starck and Sharma note that the safety profile of the devices is “compelling” and that they are used by...
Using insertable cardiac monitors (ICMs) to identify atrial fibrillation (AF) in a population at high risk for stroke guides both immediate and long-term patient management.
The findings were from an analysis of REVEAL-AF, and were published in the American Journal...
The American Heart Association has awarded research grants of more than US$14 million to four scientific teams to create a new research network focused on cardiac arrhythmias and sudden cardiac arrest.
The teams, based at Northwestern University, the University of...
A company providing what a press release calls “next-generation electrophysiology technology solutions” has announced that it now has US$170m financing. The press release reports that this includes a US$100 million Series D equity financing and a US$70 million credit...
After receiving the CE mark, Biotronik has launched its next-generation injectable cardiac monitor (ICM)—BioMonitor III—onto the European market. A press release reports that the novel device is designed to help patients with irregular heart rhythms by documenting suspected arrhythmia...
The Liverpool Centre for Cardiovascular Science, an alliance between academia and the NHS, was established to advance cardiovascular and stroke research and innovation locally, nationally, and internationally. The director of the centre, Professor Gregory YH Lip, Price-Evans chair of...
Zoll Medical, an Asahi Kasei Group Company that manufactures medical devices and related software solutions, has acquired TherOx—a company on improving treatment of acute myocardial infarction and markets systems to deliver super saturated oxygen (SSO2) therapy.
A press release reports...
A Philips press release reports both the EPIQ CVx and EPIQ CVxi now include automated applications for 2D assessment of the heart, as well as robust 3D right ventricle volume and ejection fraction measurements—with the aim of making accurate...
Data presented at Heart Rhythm 2019 (8–11 May, San Francisco, USA) and published in JACC: Clinical Electrophysiology indicates that the QDOT MICRO (Biosense Webster), designed to facilitate high power-short duration radiofrequency ablation, demonstrated safety and efficacy in achieving pulmonary...
A company providing what a press release calls “next-generation electrophysiology technology solutions” has announced that it now has US$170m financing. The press release reports that this includes a US$100 million Series D equity financing and a US$70 million credit...
Primary implantable cardioverter-defibrillator (ICD) implantation may have an incremental benefit for patients who have ischaemic cardiomyopathy without heart failure symptoms. The findings, published in Heart Rhythm, indicate that these patients have a higher risk of appropriate ICD therapy compared...
The one-year bleeding risk for patients with atrial fibrillation (AF) who undergo transcatheter aortic valve implantation (TAVI) is the same whether they are receiving vitamin K antagonist therapy or a non-vitamin K antagonist (NOAC). However, the risk of all-cause...
The HeartLight Endoscopic Ablation System is now offered at more than 100 hospitals worldwide. CardioFocus highlighted the expanded availability of its catheter ablation technology for controlled and consistent pulmonary vein isolation (PVI) in a press release.
According to the press...
Zoll Medical, which manufactures medical devices and related software solutions, has signed a definitive agreement to purchase Cardiac Science, a provider of automated external defibrillators (AEDs), related services, and accessories.
In a press release from Zoll announcing the deal, Elijah...
Stereotaxis has announced the first graduating class from its Robotic Electrophysiology Fellows Programme.
A press release from the company explains that the programme is designed to enhance the traditional electrophysiology fellowship by facilitating mastery of robotic magnetic navigation, fostering a...
Cardiologists can help to reduce the threat of nuclear war and move “the hands of the Doomsday Clock back from the midnight hour”, according to an opinion piece published in Circulation. James E Muller (Brigham and Women’s Hospital, Boston,...
The Carnation Ambulatory Monitor (Bardy Diagnostics) has received the “Best New Diagnostic Technology” award in the 2019 MedTech Breakthrough Awards programme. In a press release, Bardy Diagnostics says the device achieved the award for its innovative P-wave centric ambulatory...
The Heart Rhythm Society has published a white paper looking at the consequences of oral anticoagulants for managing stroke risk in atrial fibrillation which suggests that success with left atrial appendage closure (LAAC) device implantation may represent a “turning...
Asymptomatic heart failure patients are likely to benefit from prophylactic implantable cardioverter defibrillator (ICD) treatment, irrespective of cardiomyopathy aetiology, according to the findings from a study that assessed appropriate device therapy and mortality in New York Heart Association (NYHA)...
The American College of Cardiology (ACC) has announced the appointment of Ranna Parekh as its first director of diversity and inclusion. Parekh will work with the ACC Task Force on Diversity and Inclusion and other societies and organisations to...
Medtronic has announced US Food and Drug Administration (FDA) clearance and commercial launch for the SelectSite C304-HIS deflectable catheter system for use in procedures involving His-bundle pacing (HBP).
According to a press release, the SelectSite C304-HIS deflectable catheter system...
Valentina Kutyifa (Rochester, USA) discusses the late-breaking study findings looking at data from the family of MADIT (Multicenter Automatic Defibrillator) trials.
Kutyifa told Cardiac Rhythm News that the key takeaway from the analysis of the trials’ data was that cardiac...
Ante Anic (Split, Croatia) discusses the first in-human trial (CRYO-FIM) looking at a novel cryoballoon ablation system for pulmonary vein isolation at HRS 2019 (8–11 May) in San Francisco, USA.
Anic outlines how the cryoballoon ablation system works and notes...
Johannes Brachmann (Coburg, Germany) speaks to Cardiac Rhythm News at HRS 2019 about the REAFFIRM trial, which compared the use of rotor ablation versus conventional ablation when treating patients with persistent atrial fibrillation.
Brachmann outlines the key findings of the...
According to a press release, Boston Scientific has initiated the OPTION trial to compare safety and effectiveness of the next-generation Watchman FLX left atrial appendage closure platform to first-line oral anticoagulants (OAC)—including direct oral anticoagulants (DOAC) and warfarin—for stroke...
The Emblem subcutaneous implantable defibrillator system (S-ICD; Boston Scientific) demonstrated a high conversion efficacy and low adverse event rate. Data from the UNTOUCHED trial on the first 30 days after implantation of the device were simultaneously released at a...
CardioFocus recently announced the presentation of results from its pivotal confirmatory study evaluating the HeartLight X3 system for the treatment of AFib. The results were presented during the Heart Rhythm Society's 40th Annual Scientific Sessions (8–10 May, San Francisco,...
A trial evaluating Parasym’s neurostimulation technology shows that an ear-clip-like device can help reduce atrial fibrillation burden. The results were presented at the Heart Rhythm Society’s 40th Annual Scientific Sessions (HRS; 8–11 May) held in San Francisco, USA.
The TREAT...
Implantable cardiac devices are underused, with widespread gender and race treatment disparities, and low referral rates to electrophysiologists, according to the findings from three studies presented at the Heart Rhythm Society’s 40th Annual Scientific Sessions (HRS 2019; 8–11 May,...
Data presented at EuroPCR today indicates that renal denervation with the Symplicity system (Medtronic) is associated with a reduction in subclinical atrial fibrillation in high-risk patients with hypertension over a median follow-up of two years.
In the study, 80 patients...
Device-related thrombus (DRT) following left atrial appendage occlusion (LAAO) with the Amplatzer Amulet device (Abbott) was “infrequently” observed according to a report published in JACC: Cardiovascular Interventions (JACC: CI).
Writing in JACC: CI, Adel Aminian (Centre Hospitalier Universitaire de Charleroi,...
Highlights:
CABANA finds reduced AF and better quality of life after ablation but no reduction in mortality
ELIMINATE-AF shows low thromboembolic and bleeding events with edoxaban
Profile: Charles Antzelevitch
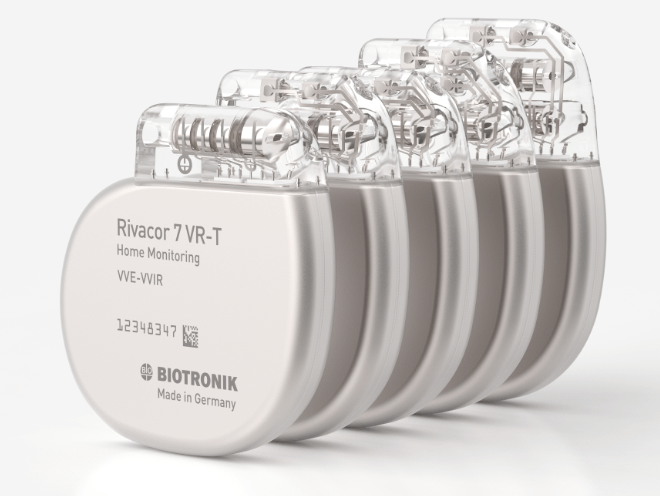
Victor Sanfins discusses his clinical experience with Biotronik's Acticor/Rivacor ICDs...
A stochastic vector-based approach to mapping and ablation of atrial fibrillation (AF) can be used to determine early sites of activation and to guide the ablation of AF drivers. Findings from targeted ablation in persistent AF using the stochastic...
The US Food and Drug Administration (FDA) has granted approval to Medtronic for the Attain Stability Quad MRI SureScan left heart lead. It is the only active-fixation left heart lead, and is designed for precise lead placement and stability,...
This advertorial has been sponsored by BIOTRONIK
Dr. Victor Sanfins is a cardiologist at Hospital da Senkora da Oliveira in Guimarães, Portugal, and a specialist in implantable electronic cardiac devices. Speaking to Cardiac Rhythm News at the 2019 annual congress...
Biotronik recently announced the full commercial launch of the Acticor device family, including Acticor DX and CRT-DX devices. Leading electrophysiologists throughout the USA are now treating patients with the new implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators...
Acutus Medical recently announced CE mark approval for AcQMap contact mapping software, offering expanded functionality that provides physicians with more options to inform individualised patient therapy. The company also announced FDA clearance of its second generation AcQMap platform. All...
In a late-breaking session at the European Heart Rhythm Association annual congress (EHRA; 17–19 March, Lisbon, Portugal), Jason Andrade of Vancouver, Canada presented the results of the CIRCA-DOSE study, a randomised clinical trial designed to evaluate the safety and...
Charles Antzelevitch is professor and executive director of the cardiovascular research program at the Lankenau Institute for Medical Research in Pennsylvania, and a professor of medicine and pharmacology and experimental therapeutics at Sidney Kimmel Medical College in Philadelphia, Pennsylvania....
The KardiaMobile device from AliveCor has received two additional Food and Drug Administration (FDA) 510(k)-cleared indications for arrhythmias that are not atrial fibrillation—for bradycardia, a resting heart rate of between 40–50 beats per minute, and for tachycardia, a heart...
An analysis on the impact of stroke severity in patients receiving the HeartWare HVAD system (Medtronic) as destination therapy shows that targeted blood pressure management helped reduce serious strokes. The HVAD system is a left ventricular assist device (LVAD)...
FEops has received CE mark approval for its FEops HEARTguide. According to a press release, FEops HEARTguide is a one-in-its-kind procedure planning environment for structural heart interventions that provides physicians unique insights to evaluate device sizing and positioning preoperatively...
The Luminize (Boston Scientific) radiofrequency balloon catheter is safe and effective at 30 days in the treatment of atrial fibrillation, data from the AF-FICIENT I study has shown. Amin Al-Ahmad (Texas Cardiac Arrhythmia Institute, Austin, TX, USA) presented the...
Phased radiofrequency (RF) ablation using the PVAC GOLD catheter for pulmonary vein isolation in paroxysmal and persistent atrial fibrillation (AF) has demonstrated good outcomes for quality of life and 12-month efficacy. Results from the multicentre GOLD-AF registry were presented by...
An app on a smartwatch can be used to detect atrial fibrillation, providing a boost for the role of digital technology in healthcare.
Mintu Turakhia and Marco Perez (Stanford University School of Medicine, CA, USA) presented the findings from the...
CardioFocus has announced the European CE Mark approval for its HeartLight X3 endoscopic ablation system, used in the treatment of atrial fibrillation.
The HeartLight X3 system is a third-generation technology that performs pulmonary vein isolation (PVI) using laser energy to create...
Researchers have identified a combination of serum biomarkers that is independently associated with higher prevalence of atrial fibrillation, and with increased incidence and recurrence of atrial fibrillation following ablation. The findings from Susana Ravassa (University of Navarra, and Navarra...
Uninterrupted anticoagulation with edoxaban (Lixiana, Daiichi Sankyo) resulted in low rates of thromboembolic and bleeding events in patients with atrial fibrillation undergoing catheter ablation, according to data from ELIMINATE AF, presented at a late breaking session at the European...
Sudden cardiac arrest remains a major cause of death among young people. Babken Asatryan outlines his research into the genetic basis of the disease, and discusses the clinical usefulness that his findings on genetic mutations could have for the...
As part of a session on novel mapping and ablation technologies of atrial fibrillation (AF) at the recent AF Symposium in Boston, USA (24–26 January), Andrea Natale gave a presentation on high power short duration ablation of AF, focusing...
Catheter ablation for atrial fibrillation leads to a reduction in atrial fibrillation, better quality of life and lower hospitalisations than medical therapy, but does not significantly reduce the risk of death, disabling stroke, serious bleeding, or cardiac arrest the...
Several treatment options are available for atrial fibrillation (AF), including pharmacological rate or rhythm control, and catheter or surgical ablation. However, consensus as to the most effective treatment remains elusive. The Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial...
Bruce Wilkoff (Cleveland Clinic, Cleveland, USA) presented results of the worldwide randomised antibiotic envelope infection prevention trial (WRAP-IT) to reduce CIED infection at the 2019 annual meeting of the European Heart Rhythm Association (EHRA; 17–19 March, Lisbon, Portugal).
The...
Boston Scientific announced data from the AF-FICIENT I study during a late-breaking clinical trial session at the 2019 European Heart Rhythm Association annual congress (EHRA; 17–19 March, Lisbon, Portugal). The data demonstrated positive safety and efficacy results with the...
In a late-breaking session at the 2019 European Heart Rhythm Association annual congress (EHRA; 17–19 March, Lisbon, Portugal), Jason Guy Andrade of Vancouver, Canada presented results of the CIRCA-DOSE study, a randomised clinical trial designed to evaluate the safety...
Boston Scientific announced it has received CE mark and initiated a limited market release of the next generation Watchman FLX left atrial appendage closure (LAAC) device in Europe.
Patients with AF are five times more likely to suffer a...
Biotronik today announced the European market release of the world’s smallest implantable cardioverter-defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) that are approved for 3 Tesla (3T) full-body MRI scans.1 According to a press release, the Acticor and Rivacor...
In a late breaking session at the 2019 AF Symposium in Boston, USA (24–26 January), it was announced that a dipole density (DD) mapping and ultrasound imaging system to identify non-pulmonary vein (PV) targets in persistent atrial fibrillation (AF)...
The commissioner of the US Food and Drug Administration (FDA), Scott Gottlieb, has unexpectedly resigned after serving just shy of two years in the post.
Gottlieb announced his intention to stand down in a letter to Alex Azar II, the...
Highlights:
Mapping dynamic patterns during atrial fibrillation can produce higher success rates
More than 40% of patients with atrial fibrillation attend the emergency department inappropriately
Profile: David Callans
Eric Prystowsky, Peter Kowey and Munveer Thind debate the CABANA trial
...
Biotronik, the University of Newcastle (Callaghan, Australia) and the Hunter New England Local Health District (HNE LHD) have partnered to shape a heart failure care model. To date, the Australian government and Biotronik have invested in the partnership.
According to a press release,...
It was recently announced that Biosense Webster has enrolled and treated the first patient in its US Investigational Device Exemption (IDE) study, which evaluates the company's QDot Micro radiofrequency (RF) ablation catheter used for the treatment of symptomatic drug-refractory...
It was announced recently that Bardy Diagnostics (BardyDx) has entered into a distribution agreement with JNC Medical, a distributor of medical technologies based in Ottawa, Canada. The agreement grants JNC Medical the right to distribute the BardyDx Carnation Ambulatory...
Patients with a previous diagnosis of atrial fibrillation who were assessed as attending the emergency department inappropriately did so because of fear, or as a result of advice from another person or self-monitoring, a study has found. Benedict Glover...
Sabine Ernst and Mattias Duytschaever are both consultant cardiologists and scientific committee members of the practical and scientific faculty of the Atrial Fibrillation Symposium, a long-standing initiative from Biosense Webster and the Johnson & Johnson Institute. Here, they talk...
Recent years have seen many advances in education for cardiologists, but little attention has been paid to the impact of new technologies, particularly digital platforms and social networking. Digital education is evolving so rapidly that most patients, clinicians, and...
Malini Madhavan (Department of Cardiology, Mayo Clinic, Rochester, Minnesota, USA) and others reported the results of a study in American Heart Journal that found that patients with atrial fibrillation who have cognitive impairment or are frail were less likely...
Ahmad Masri (Heart and Vascular Institute, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA) and others report in JACC: Clinical Electrophysiology that a systematic review and meta-analysis of the available evidence on the use of wearable cardiofibrillator therapy (WCD)...
A study carried out at the New York University Langone Health Electrophysiology Lab (New York, USA) showed significantly reduced radiation exposure levels after implementing a radiation safety time out before all electrophysiology procedures. Anthony Aizer (New York University School...
In a new financing round, Kardium has raised an undisclosed amount to fund commercial activities in Europe and Canada, and for clinical testing of the Globe system in the USA. The financing was led by funds and accounts advised by T Rowe...
A press release reports that the first patient has been enrolled in the ICE-AFIB clinical trial (AtriCure). Following Investigational Device Exemption (IDE) approval by the FDA, the first patient was treated by Niv Ad, at Washington Adventist Hospital in...
Vectorious Medical Technologies has announced the initiation of the VECTOR-HF first-in-human (FIH) clinical trial, and the successful first “in-human” implantation of the V-LAPTM monitoring device. A press release reports that the trial will enrol up to 30 patients six...
A new report from Biosense Webster sets out the scale and impact of atrial fibrillation (AF) across Europe and calls for greater awareness and understanding around the effects of AF, the need for earlier detection and diagnosis of this...
A newer type of blood-thinning medications, non-vitamin K oral anticoagulants (NOACs), is now recommended as the preferred alternative to warfarin for reducing the risk of stroke associated with atrial fibrillation (AFib), according to a focused update to the 2014...
Highlights:
"No serious adverse outcomes" with ICD shocks in young athletes playing sport
Computerised-decision support has the potential to be a "powerful tool" for preventing cardiovascular events in AF patients
Profile: Haran Burri
Richard Sutton: The first 60 years...
During his studies Haran Burri was fascinated by the simplicity of an ECG providing a vast amount of information on cardiac functions. This drew him towards a career in cardiology and electrophysiology. He is an associate professor at the...
The editors-in-chief of major cardiovascular journals—of both US and European societies—have come together to “sound the alarm” about the dangers of medical misinformation that has been disseminated through the internet, social media, and other platforms. They claim that this...
The British Heart Foundation (BHF) has announced that it is now accepting proposals for its Big Beat Challenge—a single funding award of £30 million. The goal of the Challenge, according to a press release, is to push teams of researchers...
Medtronic has announced that it has entered into a definitive agreement to acquire EPIX Therapeutics. When completed, the EPIX acquisition will expand the Medtronic cardiac ablation portfolio.
The DiamondTemp (TM) ablation system (EPIX) provides physicians with improved feedback and control...
Approval has been granted for Abbot’s TactiCath Contact Force Ablation Catheter, Sensor Enabled, which is helping more physicians integrate ablation with 3D mapping to advance the treatment of people with atrial fibrillation.
A new ablation catheter designed to help physicians...
The US government shutdown means the country’s Food and Drug Administration (FDA) cannot accept new user fees, which means the agency cannot accept new medical product applications.
FDA commissioner Scott Gottlieb took to Twitter to highlight agency employees who are...
Obstructive sleep apnoea and atrial fibrillation (AF) have presented many challenges, including identifying which patients need to be assessed for obstructive sleep apnoea and the current management options. Caroline Broughton, Shahbaz Piracha and Martin Allen write in Cardiac Rhythm...
Simultaneous monitoring of autonomic tone and ectopic activity could be used to prevent the occurrence of postoperative atrial fibrillation (POAF). Flavia Ravelli (Department of Physics, University of Trento, Povo-Trento, Italy) and the colleagues of the Cardiovascular Department of the...
Cambridge Heartwear is announcing the launch of their company and release of their Heartsense monitor in 2019. In the US, strokes are among the leading causes of death and heart disease is the leading killer. National and international data...
It has been announced that Bardy Diagnostics has monitored over 40,000 patients driven by rapid adoption of the Carnation Ambulatory Monitor (CAM), the world's first P-wave centric ambulatory cardiac patch monitor and arrhythmia detection device.
The significance of the CAM patch's P-wave focused clinical...
iRhythm Technologies has announced the publication of a new study in Nature Medicine showing expert-level detection of cardiac arrhythmias using a new deep learning, or artificial intelligence, approach for electrocardiogram (ECG) analysis across a variety of diagnostic classes.
The findings...
Premarket Approval (PMA) from the US Food and Drug Administration (FDA) has been granted for the VASCADE MVP Venous Vascular Closure System (Cardiva Medical). VASCADE MVP is the first and only vascular closure system designed and labelled specifically for...
Writing in Circulation: Cardiovascular Quality and Outcomes, David Ouyang (Stanford University, Falk Research Center, Palo Alto, USA) and others report that while the number of female first and senior authors of cardiovascular research papers has increased over the past...
BioCardia has announced its 510(k) submission for US FDA clearance of the Avance steerable introducer, which is designed for introducing various cardiovascular catheters into the heart, including via the left side of the heart through the interatrial septum. A...
Sixty years ago, the first pacemaker implant was made in Stockholm, Sweden (Senning & Elmqvist) on 8 October 1958. The patient had atrioventricular block with recurrent Adams-Stokes attacks. These were prevented by the implant—although, it only functioned for a...
The first patient has been treated in the TERMINATE AF trial (Medtronic). This is a multi-centre study evaluating two surgical ablation devices—the Cardioblate irrigated RF (IRF) system and the CryoFlex surgical ablation system—for the treatment of non-paroxysmal (persistent or...
Biosense Webster has enrolled and treated the first patient in its STELLAR US investigational device exemption (IDE) study. The study will evaluate the safety and effectiveness of Heliostar multi-electrode radiofrequency balloon ablation vatheter in treating symptomatic drug refractory recurrent...
CardioFocus has completed enrolment in a trial to evaluate its next-generation HeartLight X3 endoscopic ablation system. According to a press release, a total of 60 patients have been treated in this pivotal confirmatory trial with a one-month follow up....
A post-hoc analysis of the ICD Sports Registry—which has already indicated that many patients with implantable cardioverter defibrillators (ICD) can safely participate in vigorous or competitive sports—shows that shocks are not infrequent in young ICD patients who play competitive...
Data presented at the 2018 American Heart Association (AHA) scientific session scientific sessions (10–12 November, Chicago, USA) indicate that the use of alert-based computerised-decision support significantly increases the rate of anticoagulation prescription among patients who are hospitalised with atrial...
Quentin Fischer (Department of Cardiology, Quebec Heart and Lung Institute, Laval University, Quebec City, Canada) and others report in Circulation: Cardiovascular Interventions that patients with pre-existing left bundle branch block (LBBB) have a significantly increased risk of requiring permanent...
Abbott has announced the start of LESS-VT—the first US clinical trial to evaluate the safety and effectiveness of ablation treatment for patients with monomorphic ventricular tachycardia. The LESS-VT study, which is currently enrolling patients, will evaluate the safety and...
For Tim Betts, a career in medicine was a natural choice. After graduating he knew he wanted to be a cardiologist and, with a fascination for arrhythmias and ECGs, he became hooked on electrophysiology. His research is focused on...
Data from an open-label, pilot randomised trial (TRED-HF) indicate that if heart failure treatment is withdrawn in patients with recovered dilated cardiomyopathy, many will relapse and require treatment to be reinitiated. These findings suggest that until predictors of relapse...
The US FDA has announced plans to modernise its 510(k) clearance programme for approving medical devices for the US market. Data show that about 20% of current 510(k) devices are approved on trials that compare novel devices to predicate...
During AF Association Global AF Aware Week, Biosense Webster EMEA has published a report that uncovers the growing burden of atrial fibrillation (AF) on patients, caregivers and healthcare systems across Europe.
The new report is calling for greater awareness and...
New harmonised outcome measures for atrial fibrillation (AF) have been published in HeartRhythm. The work was funded by the Agency for Healthcare Research and Quality (AHRQ) through an interagency agreement with the Office of the Secretary Patient Centered Outcomes...
Positive 12-month data have been presented for the roll-in cohort of the pivotal CardiAMP Heart Failure Trial studying the investigational CardiAMP Cell Therapy System (BioCardia) in adult patients experiencing heart failure following a heart attack. The results were presented...
The VASCADE MVP (Cardiva Medical), a vascular closure system designed specifically for multi-access venous closure following electrophysiology procedures such as arrhythmia ablation, met its endpoints compared to manual compression in a pivotal clinical study. The AMBULATE study is the...
People who experience migraine with visual aura may have an increased risk of atrial fibrillation, according to a study published in the online issue of Neurology, the medical journal of the American Academy of Neurology.
Migraine with visual aura is...
An international study, led by researchers from the Intermountain Medical Center Heart Institute in Salt Lake City, found that a smartphone app (AliveCor) to monitor heart activity and determine if someone is having an ST-segment elevation myocardial infarction (STEMI) has...
Maisense, a start-up company specialising in stroke prevention, has recently introduced an AI-based solution that can screen stroke through early detection of atrial fibrillation (AF), that will also be presented at MEDICA (12–15 November, Düssledorf, Germany).
Patients with AF have...
EPIX Therapeutics, a medical device company that designs and manufactures a catheter-based system for the treatment of patients with atrial fibrillation (AF), has announced achievement of the enrolment goal of the DIAMOND-AF study, a global FDA-approved IDE pivotal study...
Cardiva Medical, an innovator in the field of vascular closure, has announced that new data from the randomised AMBULATE study will be presented in a late-breaking session on new trials in cardiovascular care at Scientific Sessions 2018, the annual...
The American College of Cardiology, the American Heart Association and the Heart Rhythm Society have released a guideline for the evaluation and treatment of patients with bradycardia, or a slow heartbeat, and cardiac conduction disorders.
In the guideline, bradycardia is...
Highlights:
Novel anticoagulants associated with lower all-cause mortality compared to VKAs
CABANA trial provides important new data on clinical and quality of life effects of ablation for atrial fibrillation
Profile: Tim Betts
New guidelines on CV management during pregnancy
Highlights:
Novel anticoagulants associated with lower all-cause mortality compared to VKAs
CABANA trial provides important new data on clinical and quality of life effects of ablation for atrial fibrillation
Profile: Tim Betts
New guidelines on CV management during pregnancy
Researchers at Stanford University's School of Medicine have completed enrolment of a large-scale study using the Apple Watch to determine whether a wearable technology can identify atrial fibrillation. The study rationale and design, published in the American Heart Journal,...
Cardiac Insight, Inc., a developer of wearable cardiac biosensors and clinical diagnostic software systems featuring proprietary automated data analysis algorithms, has announced its partnership with VivoSense, a company focused on integration and specialised analysis of wearable sensor data for...
The HeartMate 3 left ventricular assist device has received US FDA approval as a destination therapy for people living with advanced heart failure. With the approval, physicians can now offer the HeartMate 3 system to patients not eligible for...
A new meta-analysis of patients undergoing percutaneous closure of patent foramen ovale (PFO) after cryptogenic shock supports previous studies that indicate PFO closure is associated with an increased risk of atrial fibrillation. However, it also shows that device-related atrial...
Cardialen, a medical device company developing a low-energy implantable defibrillation therapy designed to more gently restore normal heart rhythm, has announced the closing of a US$17 million Series B investment led by RiverVest Venture Partners, along with Qiming Venture Partners,...
Medtronic has issued a software update "to address a safety risk caused by cybersecurity vulnerabilities" in 34,000 of their cardiac implantable electronic devices (CIEDs), the US FDA write in a safety communication to healthcare professionals.
Following a review of...
CathVision, an early stage medical device company developing an advanced cardiac electrophysiology recording system, CUBE, has announced that the company received ISO 13485:2016 certification for Medical Device and Quality Management Systems from Lloyd's Register.
This ISO certification indicates that the...
Adagio Medical, the developer of the iCLAS technology—the company's ultra-low temperature intelligent continuous lesion ablation system for the treatment of cardiac arrhythmias—announces that the first patients were successfully treated with its new One Shot+ cryoablation catheter. The new catheter...
Johnson & Johnson have announced that Biosense Webster has received approval from the US Food and Drug Administration (FDA) for its VISITAG SURPOINT External Processing Unit and enrolment has begun in its post-market approval study.
The VISITAG SURPOINT Module calculates...
New guidelines for the management of cardiovascular diseases during pregnancy, including upgraded recommendations of catheter ablation and risk-dependent action plans, were presented by Carina Blomström-Lundqvist, Uppsala University, Uppsala, Sweden, at the European Society of Cardiology Congress (ESC; 25–29 August,...
LivaNova has announced the first successful implantation of the Vitaria system in a patient enrolled in the Autonomic regulation therapy to enhance myocardial function and reduce progression of heart failure with reduced ejection fraction (ANTHEM-HFrEF) pivotal study. The study...
AtaCor Medical has announced that it has completed a US$8.8M Series A financing. Co-led by Boston-based Broadview Ventures and Israel-based aMoon Ventures, the financing also includes participation from a corporate partner. The investment will support the continued development of...
This article has been sponsored by Abbott
Case description
A 31 year-old female with tricuspid atresia and normally related great vessels who underwent an atriopulmonary Fontan (total cavopulmonary anastomosis) at two years of age and a subsequent Fontan revision to a...
Amit N Vora (Duke University Medical Center/the Duke Clinical Research Institute, Durham, USA) and others report in in JACC: Cardiovascular Interventions that patients who develop atrial fibrillation after undergoing transcatheter aortic valve implantation (TAVI) have a higher risk of...
Transvenous lead extraction in cardiac resynchronisation therapy (CRT) patients is not associated with increased 30-day mortality vs. non-CRT patients. The study found that age, renal impairment and sepsis were independent predictors of 30-day mortality and sepsis was the main...
Four out of ten patients with atrial fibrillation but no history of stroke or transient ischaemic attack have previously unknown brain damage, according to the first results of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF) presented at European Society...
Atricure have announced that the enrolment of the full cohort of 153 patients in the CONVERGE IDE clinical trial has been completed. CONVERGE IDE is the first prospective, randomised study comparing the Convergent approach to endocardial catheter ablation in persistent...
New findings from the CRYO4PERSISTENT AF clinical trial demonstrate improved quality of life, reduced symptoms from abnormal heart rhythms, and low incidence of reinterventions and repeat ablation procedures. The study evaluated patients with symptomatic persistent atrial fibrillation (AF) treated...
Biotronik is now the exclusive US distributor for InfoBionic's MoMe Kardia external cardiac diagnostic monitor, it has announced in a press release. The device benefits patients suspected of experiencing cardiac arrhythmias and is designed to increase early detection and...
Thomas S Gilhofer (University Heart Center, Department of Cardiology, University Hospital Zurich, Zurich, Switzerland) and others report in Structural Heart that a combined procedure involving both transcatheter aortic valve implantation (TAVI) and left atrial appendage closure is a feasible...
At the 2018 European Society of Cardiology (ESC) Congress (Munich, Germany), British Heart Foundation (BHF) announced the “Big Beat Challenge”—an £30m award that seeks to bring together world-leading researchers and innovators to identify and solve any of the biggest...
At the 2018 European Society of Cardiology (ESC) Congress (25–29 August, Munich, Germany), Siemens Healthineers launched Magnetom Sola Cardiovascular Edition—a 1.5 Tesla magnetic resonance imaging (MRI) scanner that is specifically designed for cardiovascular examinations. A press release reports that...
WeHealth, by Servier, has launched Cardioskin—a connected solution that is designed to function as an ambulatory wearable 15-lead ECG—at the 2018 European Society of Cardiology Congress (25–29 August, Munich, Germany). A press release reports that Cardioskin is composed of...
Royal Philips has introduced the EPIQ CVx cardiovascular ultrasound system. Built on the powerful EPIQ ultrasound platform, EPIQ CVx is specifically designed to increase diagnostic confidence and simplify workflow for clinicians, giving them more time to interact with their...
More than 100,000 people have been trained in hands-only cardiopulmonary resuscitation (CPR) since the American Heart Association (AHA) launched its hands-only CPR training kiosk programme in 2016. As part of the programme, that is supported by Anthem Foundation in...
A feasibility study of a novel left atrial appendage device (Ultraseal, Cardia) indicates that the device is associated with a high rate of procedural success and a fast learning curve. It also suggests that the device has an extremely...
BioSig Technologies have announced that the Company has received 510(k) clearance for its first product, PURE EP System, from the US Food and Drug Administration (FDA).
The non-invasive PURE EP System is a computerised system intended for acquiring, digitising, amplifying,...
Milestone Pharmaceuticals, a clinical-stage cardiovascular company, announced that the first patient has been randomised in its phase 3 clinical study of etripamil. Etripamil is a new investigational, rapid-onset, short-acting calcium channel blocker administered intranasally by the patient designed to...
Medtronic plc have announced the start of a pilot study of its investigational extravascular implantable cardioverter defibrillator (EV ICD) system, in which a lead is placed outside of the heart and veins to deliver lifesaving defibrillation and antitachycardia pacing...
A new study, published in JAMA Network Open, indicates that patients with atrial flutter have a lower incidence of stroke than patients with atrial fibrillation who have the same CHA2DS2-VASc score. Given current European guidelines advise that patients with...
VisCardia has announced the first implant of its VisONE implantable system for heart failure, and the commencement of its VisONE Heart Failure pilot study in Ukraine. According to a press release, the VisONE implantable system delivers VisCardia’s proprietary asymptomatic...
How to best combine optimal screening and diagnosis for atrial fibrillation (AF) has long been a challenge. At European Heart Rhythm Association Congress (EHRA; 18–20 March 2018, Barcelona, Spain) Jens Eckstein (University of Basel, Basel, Switzerland) presented the case...
A study looking at patients undergoing lead extraction found that they were more likely to survive superior vena cava tears when treatment included the Bridge occlusion balloon. Lead author, Roger Carrillo (University of Miami, Miami, USA) concluded that when...
Cyber security has become a hot topic in recent years. Leslie Saxon writes in Cardiac Rhythm News about the cybersecurity risks for implantable cardiac devices.
In the past year, the US FDA and a division of Homeland Security that responds...
The American Heart Association (Association) and Laerdal Medical (Laerdal) are furthering their decades-long alliance to deliver a new standard of resuscitation quality and patient care centred on CPR competence. Attendees representing 30 of the nation’s largest health care systems...
MEDICALgorithmics and US subsidiary Medi-Lynx Cardiac Monitoring L.L.C., have announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance for the PocketECG Cardiac Rehabilitation System (CRS), a new mobile cardiac rehabilitation system designed to provide high-quality...
A phase 3 trial of Zoll Medical’s LifeVest has missed its primary endpoint. The study found that people who started using the wearable defibrillator the week after they had a heart attack did not experience a statistically significant reduction...
Boston Scientific has signed an agreement to acquire Claret Medical, which developed and commercialised the Sentinel cerebral embolic protection system. The device is used to protect the brain during certain interventional procedures, predominately in patients undergoing transcatheter aortic valve...
Medtronic has announced the first enrolments in a new clinical trial evaluating the ECG (electrocardiography) Belt Research System (ECG Belt) as a diagnostic tool for optimising cardiac resynchronisation therapy (CRT) for patients with heart failure. The first patient enrolled...
The link between cardiac diseases and stroke is well known and reported. Wolfram Döhner writes in Cardiac Rhythm News about the needs for interdisciplinary thinking and collaboration.
Stroke is a leading cause of death and the main cause of physical...
His bundle pacing was successful in 92% of patients and the primary endpoint of death, heart failure hospitalisation or upgrade to biventricular pacing was significantly reduced in the His bundle pacing group. Right ventricular pacing is associated with heart...
Symptomatic atrial fibrillation (AF) patients with previous failed catheter ablation or structural changes associated with higher recurrence of AF could benefit from thoracoscopic ablation and left atrial appendage excision. The thoracoscopic approach was associated with higher initial rates of...
Bardy Diagnostics has announced that it has raised additional growth capital from its existing equity investors, including SV Health Investors ("SVHI"), Health Enterprise Partners, and Ascension Ventures. The growth capital will be used to expand BardyDx's sales force and...
The mHealth Screening To Prevent Strokes (mSToPS) randomised clinical trial showed that home-based wearable electrocardiogram (ECG) sensor patch monitoring, compared with delayed monitoring, resulted in a higher rate of atrial fibrillation (AF) diagnosis after four months. Wearing an ECG patch...
Differences between men and women mean that outcomes after cardiac events can differ between the sexes. Cecilia Linde writes in Cardiac Rhythm News about a new consensus document that looks to address these issues.
There is an increasing awareness that...
A press release has announced US Food and Drug Administration (FDA) labeling expansion for the Medtronic SelectSecure MRI SureScan Model 3830 cardiac pacing lead to include stimulation of the bundle of His. It is the only pacing lead on...
A press release reports that the CALYPSO programme will receive 14 million euros to develop CorWave Neptune—a new type of cardiac support designed to improve the management of patients with severe heart failure—as part of the Programme d’Investissements d’Avenir(PIA)...
Medtronic has received United States Food and Drug Administration (FDA) approval for a less-invasive implant approach of its HVAD System, a left ventricular assist device (LVAD) for patients with advanced heart failure. The HVAD System is the smallest commercially...
Black people who survive cardiac arrest during hospitalisation have lower odds of long-term survival compared with similar white survivors, according to new research published in Circulation.
Half the difference in one-year survival rates, however, remained unexplained. Nearly one-third of the racial...
CorWave has announced that it has obtained patent no. US 9,968,720 B2 on May 15, 2018 from the US Patent and Trademark Office. Titled “Implantable pump system having an undulating membrane,” according to a press release, this patent describes...
Research finds use of smaller, self-contained devices reduced complications for up to 18 months compared with conventional designs
The findings of the research, led by Ohio-based Cleveland Clinic, appeared in the journal Heart Rhythm. The study compared patient outcomes for...
CorWave obtained patent no. US 9,968,720 B2 on May 15, 2018 from the United States Patent and Trademark Office.
Titled “Implantable pump system having an undulating membrane,” the patent describes the application of CorWave’s technology for blood pumping in a...
This article was sponsored by Abbott.
Left ventricular assist devices (LVAD) are mechanical pumps that are implanted inside a person's chest to help their weakened heart ventricle pump blood throughout the body. The mechanical device supports the left heart chamber...
As part of its plan to expand it atrial fibrillation ablation therapy offering, Boston Scientific Corporation has announced a definitive agreement to acquire Cryterion Medical, a privately-held company developing a single-shot cryoablation platform for the treatment of atrial fibrillation...
Investigators at Children's Hospital Los Angeles and the University of Southern California, Los Angeles, USA, have demonstrated the feasibility of implanting a micropacemaker system in the pericardial sac surrounding the heart — a breakthrough that may open up new cardiac pacing...
By Hannah Woolley
Findings from the Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease (COMPASS) trial suggest that the combination of low-dose rivaroxaban and aspirin significantly lowers the incidence of both major adverse cardiovascular...
The annual census of UK consultants and higher speciality trainees—Focus on Physicians 2017–18—indicates that more than half of all consultants and two thirds of trainees reported frequent gaps in trainees’ rotas, with one in five respondents saying these are...
By Hannah Woolley
In a multivariate analysis including both BRUISE CONTROL 1 and BRUISE CONTROL 2 patients and adjusted for antiplatelet use, there was no difference in clinically significant haematoma between direct oral anticoagulants (DOACs) and warfarin.
Oral anticoagulant use is...
By Hannah Woolley
Longer dwell times are related to higher rates of complication during lead extraction. There have been various studies looking at the risk factors for transvenous lead extraction but there is limited knowledge about extraction of leads that...
Patients with scar-related ventricular tachycardia (VT) do not often achieve a cure. Jason S Bradfield and Kalyanam Shivkumar write in Cardiac Rhythm News about how to tackle this issue.
Success with medical therapy or catheter ablation may involve long periods...
HeartSciences has announced that it has been highlighted as a “Start-Up to Watch” in the June 8, 2018 issue of the MedTech Strategist. The article titled “Heartsciences: artificial intelligence improves frontline risk stratification for heart diseases” discusses HeartSciences’ role...
Wearable cardioverter defibrillators – vest-like devices that deliver electric shocks to interrupt a dangerous heart rhythm – may be a safe and effective alternative to surgically implanted devices in children with ventricular heart rhythm disorders that put them at risk for...
Catheter ablation for atrial fibrillation (AF) produced no significant improvement in death, disabling stroke, serious bleeding, or cardiac arrest but did reduce death or cardiovascular hospitalization and recurrent AF in the Catheter Ablation versus Antiarrhythmic Drug Therapy (CABANA) trial,...
Published in the heart journal Europace, the researchers found that a 10% loss in weight along with management of associated risk factors, can reverse the progression of the disease. They studied 355 overweight or obese people who lost varying amounts...
New guidance regarding the selection and evaluation of wearable devices for use in regulatory trials and to support labelling claims has been published in the journal Value of Health. The report, Selection of and Evidentiary Considerations for Wearable Devices and...
The US Food and Drug Administration (FDA) released the following two draft guidance documents: These guidance documents provide industry and FDA staff with recommendations on the least burdensome means of assessing the performance of catheters, guidewires, and delivery systems submitted...
The first-in-human phase I trial will take place in Australia for Verseon’s PROAC (PRecision Oral AntiCoagulant) VE-1902, which has successfully completed regulatory toxicology studies and was well-tolerated in 28-day repeat dosing.
PROACs are a novel class of anticoagulants that show...
A new study has found that the more alcohol you drink, the higher your heart rate gets. These finding were presented at European Heart Rhythm Association Congress (EHRA; 18–20 March 2018, Barcelona, Spain).
The Munich Beer Related Electrocardiogram Workup (MunichBREW)...
The trials found that ultralow temperature cryoablation is feasible and shows excellent safety and efficacy. The clinical outcomes for flutter ablation show a 94% success rate at three months.
Ultralow temperature ablation uses near-critical nitrogen. The catheter operates at the...
The algorithm (Kardiaband, AliveCor) for atrial fibrillation (AF) detection, when supported by physician review can accurately differentiate AF from sinus rhythm (SR). This technology can help screen patients prior to elective cardioversion and avoid unnecessary procedures.
One hundred patients were...
The American Heart Association has announced research grants totalling more than US$28 million to the scientific teams that will create a new research network focused on understanding the causes of atrial fibrillation (AFib). The new knowledge they discover will...
BIOTRONIK have announced the opening of its Education and Innovation Centre in Ebisu, Tokyo, Japan. The centre will provide the latest educational programs and services for medical professionals and respond to the needs of medical practitioners through efficient information...
The American College of Cardiology (ACC), Heart Rhythm Society (HRS), North American Society for Cardiovascular Imaging (NASCI), Society for Cardiovascular Angiography and Interventions (SCAI), and the Society for Computed Tomography (SCCT) have published, in the Journal of the American...
Zenicor Medical Systems AB has been selected as sole supplier for a screening programme in the UK for atrial fibrillation. The screening programme is the world’s largest randomised controlled trial to discover whether screening systematically for atrial fibrillation, a...
Royal Philips has announced that it has signed an agreement to acquire EPD Solutions. EPD’s cardiac imaging and navigation system helps electrophysiologists navigate the heart by generating a detailed 3D image of the cardiac anatomy, while also pinpointing the...
The United States Food and Drug Administration (FDA) has classified Medtronic recent voluntary urgent field action related to the HeartWare HVAD System unexpected power source switching as a Class I recall. Class I recalls describe situations where there is...
The total procedure-related complications after surgical minimally invasive pulmonary vein isolations (MIPI) were higher; this was mainly due to more major complications. During a two-year follow-up there were three transient ischaemic attacks or cerebrovascular accidents after MIPI compared to...
Researchers will use a European network of 90,000 patients, including 20,000 DNA samples, to look at different approaches for prevention and treatment of sudden cardiac arrest for men and women.
Sudden cardiac arrest is the consequence of the heart rhythm...
Apixaban and warfarin are equally safe during catheter ablation of atrial fibrillation, according to results of the AXAFA-AFNET 5 (Anticoagulation using the direct factor Xa inhibitor apixaban during Atrial Fibrillation catheter Ablation: Comparison to vitamin K antagonist therapy) (German...
The Copenhagen City Heart Study set out to investigate whether left atrial (LA) functional measures predict atrial fibrillation (AF) in the general population. They found that LA functional measures predict AF in the general population and can provide prognostic...
The first systematic evaluation of coagulation point-of-care testing in edoxaban treated patients has been carried out. The results were presented by Florian Härtig, University of Tuebingen, Germany, at the International Stroke Conference (ISC; 24–26 January 2018, Los Angeles, USA)
Currently...
The interim results of the REAFFIRM (Randomised Evaluation of Atrial Fibrillation treatment with Focal Impulse and Rotor Modulation guided procedures) trial showed there was no statistically significant difference in outcomes between the treatment and control arms. These results were...
Octreotide therapy has the potential to offer atrial fibrillation (AF) patients with arteriovenous malformations (AVM) related gastrointestinal (GI) bleeding another treatment option. It is a safe way to reinitiate oral anticoagulants (OACs) by bringing down the risk of repeat...
One-year results from the Dielectric Unravveling of RAdiofrequency ABLation Effectiveness (DURABLE-I) clinical trial have shown that the KODEX (Navix International Limited) system help to improve effectiveness by detecting gaps in real time during the index ablation procedure. The results...
The results of the WATCH AF trial, the world’s first international, prospective and double-blinded clinical trial (ClinicalTrials.gov ID: NCT02956343) evaluating the accuracy of a smartwatch to detect atrial fibrillation were announced at the European Heart Rhythm Association Congress. (EHRA...
Dhanunjaya Lakkireddy is executive medical director at The Kansas City Heart Rhythm Institute at HCA Midwest Health, Kansas, USA. He holds the title Professor of Medicine, is board certified in Cardiology and Electrophysiology and is a renowned electrophysiologist. He...
Luigi Di Biase is a prominent electrophysiologist, section head of Electrophysiology, director of Arrhythmia Services, and professor of Medicine (Cardiology) at the Albert Einstein College of Medicine, New York, USA. In addition, he serves as senior researcher at the...
Highlights:
Apixaban is a safe alternative to warfarin during catheter ablation of atrial fibrillation and may have a positive effect on cognitive function
Stroke prevention drugs may help reduce dementia risk for atrial fibrillation patients, consensus document finds
Leslie...
Highlights:
Apixaban is a safe alternative to warfarin during catheter ablation of atrial fibrillation and may have a positive effect on cognitive function
Stroke prevention drugs may help reduce dementia risk for atrial fibrillation patients, consensus document finds
Leslie...
Boston Scientific Europe has announced the launch of the HeartLogic Heart Failure Diagnostic in Europe. With this launch, the first and only diagnostic tool that enables proactive heart failure (HF) care is now available for patients in select countries...
iRhythm Technologies has announced results of a study which utilised Zio by iRhythm, an extended continuous cardiac monitoring system, to provide a comprehensive picture of the burden of atrial fibrillation (AF) in patients. Utilising this data in combination with...
Results from a research study demonstrating the feasibility of a novel approach to delivering pacing and defibrillation therapy in which a lead is placed under the sternum (breastbone), outside of the heart and veins have been announced. Data from...
Study results show that the AdaptivCRT algorithm is associated with improved patient survival. The real-world, prospective registry of 1,835 patients, use of the AdaptivCRT algorithm was associated with a 31% relative reduction in all-cause mortality compared to conventional cardiac...
Results from an analysis of the LATITUDE database which evaluated the successful reduction of inappropriate shocks using the SMART Pass sensing filter in patients implanted with the EMBLEM Subcutaneous Implantable Defibrillator (S-ICD) System have been announced. The real-world data...
The AngelMed Guardian System is an implantable cardiac monitor intended to detect and alert patients of a potential heart attack.
The Guardian System consists of three components:
The Implantable Medical Device (IMD), which monitors the heart's electrical activity (electrograms)...
Two-year results show rivaroxaban was associated with reduced stroke and systemic embolism versus warfarin, without altering risk of major bleeding. The study evaluated efficacy and safety of rivaroxaban, apixaban and dabigatran each versus warfarin.
The Janssen Pharmaceutical Companies of Johnson...
The US FDA has issued a class I recall due to a malfunction in the device’s outflow graft assembly that may cause the outflow graft to twist and close up (occlusion) over time. Occlusion of the outflow graft can...
Jagmeet Singh is the founding director of the Resynchronisation and Advanced Cardiac Therapeutics Program. Singh is the principal investigator for the Personalised CRT-MPP Post-Approval Study and a paid consultant for Medtronic. In this commentary he discusses his research on...
The American Heart Journal has published the results of a head-to-head comparison of two patch-based arrhythmia monitoring systems. The study, "Comparison of two ambulatory patch ECG monitors: The benefit of the P-wave and signal clarity" concluded that the BardyDx...
Investigators from the Mayo Clinic and AliveCor demonstrated that a trained artificial intelligence network can help identify people with increased risks of arrhythmias and sudden cardiac death, despite displaying a normal heart rhythm on their electrocardiogram.
Up to half...
The SMART Pass sensing filter (Boston Scientific), when in patients implanted with the EMBLEM Subcutaneous Implantable Defibrillator (S-ICD) System, successfully reduces inappropriate shocks. This is the result of an analysis of the LATITUDE database, presented during a late-breaking clinical...
Cardiac contractility modulation (CCM) in heart failure is safe, the results from the FIX-HF-5C randomised controlled trial demonstrate, with all primary and secondary safety endpoints met.
The results were presented by William Abraham (Internal Medicine, Division of Cardiovascular Medicine,...
An investigational algorithm, utilising the accelerometer signal in the Micra Transcatheter Pacing System (TPS) (Medtronic) may restore AV synchrony, improving cardiac function in patients with sinus rhythm and atrioventricular (AV) block, the results of a new clinical study show.
The...
The first patient has been enrolled and treated in the QDOT AF study (Biosense Webster). The study will evaluate the delivery of high power, short duration ablation with QDOT MICRO, a novel radiofrequency (RF) ablation catheter for the...
Abbott has announced US Food and Drug Administration (FDA) clearance of the Advisor HD Grid mapping catheter, sensor enabled. Advisor HD Grid employs a new design that allows physicians to see things differently, capturing and analysing data in a novel...
This article has been sponsored by Boston Scientific.
Case description
A 62-year-old woman, with recent sinus venosus atrial septal defect correction surgery, developed a persistent macroreentrant atrial tachycardia and macroreentrant atrial tachycardia (MAT) ablation was performed. The diagnosis of this congenital...
Acutus Medical has announced that the AcQMap High-Resolution Imaging and Mapping System has been utilised for the first time in US patients. The company also revealed initiation of a new clinical study to evaluate the technology during atrial fibrillation...
Preventice Solutions has announced the launch of the BodyGuardian Mini, the smallest, reusable long-term Holter technology available for cardiac monitoring. The new, compactly designed technology includes an ultra-small, lightweight cardiac monitor that enables up to 14-days of ECG measurements...
The US Food and Drug Administration (FDA) has granted approval for the HeartLight Excalibur Balloon, a next-generation technology designed for the treatment of paroxysmal atrial fibrillation (PAF).
The Excalibur Balloon leverages the proven universal balloon design of the company's FDA-approved HeartLight Endoscopic Ablation System and...
The CARTO VIZIGO Bi-directional Guiding Sheath is now available in the United States. This is the first commercially available steerable guiding sheath that can be visualised on the CARTO 3 System during a catheter ablation procedure, helping electrophysiologists reduce dependency...
Stereotaxis has announced a strategic collaboration to integrate the Stereotaxis Niobe Magnetic Navigation System and the Acutus Medical AcQMap High Resolution Imaging and Mapping System with the goal of improving patient care and the physician experience in electrophysiology.
“I was...
At the AF Symposium (Orlando, USA) John Camm, St George’s University of London and Imperial College, London, UK, looked at the current status of screening for atrial fibrillation (AF). This is as an area of controversy with practitioners on...
The HAVOC score was developed following the CRYSTAL-AF study, to see if there was an easier way to identify which patients with atrial fibrillation were most at risk of stroke. Susan Zhao, Santa Clara Valley Medical Center, San Jose,...
More patients than ever before are being given NOACs due to their relative stability and safety, but the risk of gastrointestinal bleeding with NOACs is higher than with warfarin. This has led researchers to look for new ways of...
Results from a multicentre study of catheter ablation guided by DEEP (decrement-evoke potential) mapping show that using DEEP mapping meant that ablation was successful in the majority of patients and they could no longer be induced into clinical ventricular...
Medical devices, including cardiovascular implantable electronic devices could be at risk for hacking. In a paper published in the Journal of the American College of Cardiology, the American College of Cardiology’s Electrophysiology Council examines the potential risk to patients...
Local activation mapping at sites of termination of persistent atrial fibrillations (AF) shows repetitive patterns including rotational or focal activity reports a study in Circulation: Arrhythmia and Electrophysiology.
Therapy for persistent atrial fibrillation is limited by uncertainty about sustaining mechanisms....
It was a year of big results for catheter ablation, continuing the procedure’s steady rise in atrial fibrillation (AF) management. Mark O’Neill, consultant cardiologist and professor of Cardiac Electrophysiology at St Thomas’ Hospital and King’s College London, UK, spoke...
Nassir Marrouche is a prominent electrophysiologist, executive director of the Comprehensive Arrhythmia Research and Management (CARMA) Centre at the University of Utah in Salt Lake City, USA, and director of the Western Atrial Fibrillation Symposium (23–24 February 2018, Park...
Highlights:
Real-time lesion formation and gap detection during ablation: DURABLE-I follow-up
Octreotide enables left atrial appendage closure in AF patients with GI bleeding
M Edip Gurol: Brain MRI scans can inform the choice between OACs and LAA closure for non-valvular...
LivaNova has finalised the US$190 million sale of its cardiac rhythm unit to MicroPort Scientific. The final terms of the deal commit LivaNova to paying up to US$20 million in indemnity linked to a probe initiated by antitrust officials around...
The US FDA has approved a firmware update that is now available and is intended as a corrective action (recall), to reduce the risk of patient harm due to premature battery depletion and potential exploitation of cybersecurity vulnerabilities for...
A medical device company has announced the results of a clinical study serially conducted at The Icahn School of Medicine, Mount Sinai Hospital, New York, USA and the West Virginia University (WVU) Heart and Vascular Institute, Morgantown, USA. The...
Murj, a healthcare technology company focused on transforming implantable cardiac device management, has announced the availability of OnSite— the industry’s first cloud-based workflow solution for in-office cardiac device management.
OnSite is an extension of Murj’s proven workflow for remote transmission...
There is a disparity between indications for and utilisation of implantable cardioverter defibrillators (ICDs) in Asian patients with heart failure, a recent study finds. The results come from a study authored by Yvonne May Fen Chia et al, recently...
A recent study by Marc P Waase (Columbia University Medical Center, New York, USA) et al published in the Journal of the American Medical Association (JAMA) found that abnormal electrocardiograms (ECGs) are common among professional basketball players in the...
Patient safety is the focus of newly published updates to consensus statements that address the use of antithrombotic drugs by individuals who may require regional anaesthesia or interventions targeting acute or chronic pain.
The lengthy statements, published in tandem in...
This video has been sponsored by LivaNova.
Sleep apnoea is a major chronic disease that can have cardiovascular consequences. Around 40–50% of people with atrial fibrillation (AF) have obstructive sleep apnoea, rising to around 60% in patients with implantable cardiac...
Boston Scientific have announced the acquisition of Securus Medical Group, a privately-held company that has developed a thermal monitoring system for the continuous measurement of oesophageal temperature. Boston Scientific has been an investor in Securus since 2016, and the...
Cardiac health apps and wearable clinical technology is on the rise, along with remote monitoring and wireless connection functionality in implantable cardiac devices. While the amount of clinical trials and published papers on new technology in this field is...
Wendy Tzou (University of Colorado School of Medicine, Denver, USA) and colleagues at the International Ventricular Tachycardia Ablation Center Collaborative group (IVTCC) published two papers (in Heart Rhythm and Circulation: Arrhythmia and Electrophysiology) in 2017 on the potential impact...
M Edip Gurol is a stroke neurologist with particular expertise in the care of patients at high risk for ischaemic (blockage type) strokes and haemorrhages. His research focuses on clarifying the mechanisms of brain small vessel diseases that increase...
Two real-world analyses featuring the AdaptivCRT algorithm reinforce that its use is linked to a reduction in atrial fibrillation (AF) episodes, as well as tied to higher patient activity levels. The results, involving 408 patients with heart failure and...
One-year results from the CRYO4PERSISTENT AF study of ablation with the Arctic Front Advance Cryoballoon to isolate the pulmonary veins in patients with symptomatic persistent atrial fibrillation (AF). The Arctic Front Advance Cyroablation System is not approved for treating...
The heart is capable of terminating arrhythmias itself after local gene therapy, potentially avoiding the need for patients to undergo painful electric shocks, according to a proof-of-concept study presented at EHRA 2018, a European Society of Cardiology congress.
Atrial fibrillation...
Patients with atrial fibrillation could reduce the risk of dementia by taking stroke prevention medications, according to recommendations published online in EP Europace1, a European Society of Cardiology journal, and presented at EHRA 2018.2 The international consensus document was...
Data from the TRUE-HD study has been announced during a late-breaking clinical trial session at the annual congress of the European Heart Rhythm Association (EHRA) in Barcelona, Spain. The data demonstrated the RHYTHMIA Mapping System, when paired with the...
Obese patients are at a higher risk of failed first subcutaneous implantable cardioverter-defibrillator (S-ICD) shocks during defibrillation testing. This is what a group from the University of Pennsylvania, CorVita Science Foundation and Boston Scientific Corporation, all USA, found. The...
Low level of daily physical activity was associated with a higher risk of atrial arrhythmias in heart failure patients with implantable devices. A low level of baseline physical activity was also associated with a higher risk of death of...
The first patient has been enrolled and treated in the SHINE clinical study in Europe. The multicentre study aims to evaluate balloon ablation catheter for safety and effectiveness in achieving pulmonary vein isolation in treatment of paroxysmal atrial fibrillation.
The...
Interim data from 228 patients (of which 132 were adjudicated for efficacy) showed that AndexXa rapidly and significantly reversed anti-Factor Xa activity (the anticoagulant mechanism of these drugs) when administered as a bolus, and sustained this reversal when followed...
New late-breaking clinical trial data from the MOMENTUM 3 clinical study shows overall survival of 83% at 2-years and marked improvement in clinical outcomes for our patients suffering with advanced heart failure. The data were published online in The New...
EBR Systems has announced enrollment of the first patients in the global SOLVE-CRT (Stimulation of the Left Ventricular Endocardium for Cardiac Resynchronisation Therapy) clinical trial.
The first patients were enrolled by Christian Butter at Immanuel Klinikum Herzzentrum Brandenburg in...
iRhythm Technologies has announced primary results of the mHealth Screening to Prevent Strokes (mSToPS) study that evaluated detection of silent atrial fibrillation (AF) in high-risk individuals using Zio by iRhythm.
Electrocardiogram (ECG) recording and analysis were carried out using the...
Fysicon has announced that it has been acquired by Canon Medical Systems Corporation (Otawara, Japan).
Linda Elberse, chief executive officer of Fysicon said, "Being part of a major player as Canon Medical Systems Corporation gives us the opportunity to...
AblaCor medical corporation has announced that it has received a notice of allowance from the United States Patent & Trademark Office on five additional patents for its CircumBlator AFib catheter ablation system to advance pulmonary vein isolation ablation procedures for patients with atrial...
Survival from cardiac arrest doubled when a bystander stepped in to apply an automated external defibrillator (AED) before emergency responders arrived, according to new research published in the journal Circulation.
Researcher looked at 49,555 cardiac arrests across the USA and...
CardioFocus have announced the initiation of a clinical evaluation of the new HeartLight X3 system for the treatment of AF.
Building upon the advanced features of the current HeartLight endoscopic ablation system—direct tissue visualisation, titratable laser energy, and compliant balloon technology—HeartLight X3 is...
Medtronic is recalling certain implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators (CRT-Ds) due to a defect in the manufacturing process. This defect causes an out of specification gas mixture inside the device and may prevent the device...
EPD’s Cardiac Mapping and Navigation System has received CE mark approval. The system helps electrophysiologists navigate the heart by generating an accurate 3D map, while imaging and pinpointing the exact location and orientation of catheters in the heart during...
AtriCure, Inc., a leading innovator in treatments for atrial fibrillation and left atrial appendage management, has announced that it has launched the AtriClip FLEX•V Left Atrial Appendage (LAA) Exclusion System in the United States. The new AtriClip FLEX•V is...
The FDA is advising caution before prescribing the antibiotic clarithromycin (Biaxin) to patients with heart disease because of a potential increased risk of heart problems or death that can occur years later. The FDAs recommendation is based on a...
Felix Weise (Cardioangiologisches Centrum Bethanien, Frankfurt, Germany) and others report in EuroIntervention that a strategy of six weeks’ duration of dual antiplatelet therapy (DAPT) followed by lifelong aspirin “appears to be a viable alternative” to existing antithrombotic regimens in...
New data from the EXCEL trial, which found that percutaneous coronary intervention (PCI) was non-inferior to coronary artery bypass grafting (CABG) at three years in patients with left main disease, indicate that CABG patients have a significantly increased risk...
Cardiva Medical, an innovator in the field of vascular closure, has announced that the company has closed on US$11 million in additional financing – bringing total equity and debt financing in the current round to US$41 million. The additional financing...
The Food and Drug Administration (FDA) has today issued the final rule on “human subject protection; acceptance of data from clinical investigations for medical devices”.
The rule updates the FDA’s standards for accepting clinical data from clinical investigations conducted...
A miniature leadless pacemaker was safe and effective at three years in the first-in-human LEADLESS study, according to a research letter published in Circulation. However, one patient experienced a complication related to battery malfunction, which led to a battery advisory...
African Americans with atrial fibrillation (AF) have a significantly higher risk of stroke than Caucasians with the condition, according to new research published in HeartRhythm by researchers from the Perelman School of Medicine at the University of Pennsylvania, USA. The new findings...
A collaborative study, has found that older patients with atrial fibrillation (AF) undergoing cardiac surgery accompanied with surgical left atrial appendage closure had a lower risk of readmission for thromboembolism over the following 3 years. The study was carried...
Biosense Webster, a Division of Johnson & Johnson Medical NV/SA, and a leader in the diagnosis and treatment of cardiac arrhythmias, recently presented new data evaluating the one-year outcomes of atrial fibrillation (AF) ablation using the CARTO 3 System CARTO...
Written by Hannah Woolley, Cardiac Rhythm News Editor
Heart imaging has been successfully used to predict the benefit or futility of catheter ablation. The investigators described their findings in the Journal of the American College of Cardiology: Cardiovascular Imaging.
Around 20–30% of...
Philips is recalling the HeartStart MRx defibrillator due to a defect in the device's gas discharge tube. The gas discharge tube has micro cracks which allows internal gasses to escape and causes the tubes to not function as expected....
A recent study by Gry Haaland (University of Bergen, Bergen, Norway) et al published in the Journal of the American Association of Medicine (JAMA): Internal Medicine points to a possible association between warfarin use among patients older than 50...
Acesion Pharma, a Danish biotech company developing novel treatments for atrial fibrillation (AF), the most common cardiac arrhythmia, announced it has received approval to commence its first clinical study for its lead compound AP30663. The phase 1 study in...
Highlights:
Less bleeding in ablation with interrupted dabigatran compared to uninterrupted warfarin
Successful ablation for ventricular tachycardia associated with markedly reduced mortality in advanced heart failure patients
Natalia Trayanova: Imaging-based simulations for predicting sudden death and guiding ablation
Nassir Marrouche: Profile
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2018/02/Cardiac-Rhythm-News-issue-39-low-res.pdf
Respicardia, Inc., a medical technology company developing therapies that improve cardiovascular health, have announced the first U.S. commercial case using the remedē system to treat central sleep apnoea was completed at The Ohio State University Wexner Medical Center in...
Natalia Trayanova envisions the future of cardiology and electrophysiology advancing through personalised medicine and computational simulations. Trayanova is the director of the Computational Cardiology Lab at Johns Hopkins University (Baltimore, USA), as well as the Murray B Sachs professor...
Local activation mapping at sites of termination of persistent atrial fibrillations (AF) shows repetitive patterns including rotational or focal activity reports a study in Circulation: Arrhythmia and Electrophysiology1.
Therapy for persistent atrial fibrillation is limited by uncertainty about sustaining mechanisms. ...
The results of the CASTLE-AF study, published today in the New England Journal of Medicine, show that patients treated with radiofrequency catheter ablation rather than traditional drug therapies for atrial fibrillation had improved outcomes.
“This clinical trial is the first time...
Following EHRA’s decision to hold its own annual congress in March and the difficulty for the industry to financially support Cardiostim as well as before, the decision has been taken no to hold Cardiostim 2018.
In spite of the extraordinary...
Boston Scientific Corporation today announced it has closed an investment and entered into an acquisition option agreement with Millipede, Inc., a privately-held company that has developed the ring system for the treatment of severe mitral regurgitation (MR).
Under the terms of the...
The results of ABRIDGE-J, a Japanese multicentre randomised study on minimally interrupted dabigatran vs. uninterrupted warfarin for catheter ablation, were presented during a late-breaking trials session at the American Heart Association (AHA) Scientific Sessions (11–15 November 2017, Anaheim, USA)....
Imricor Medical Systems announced today the completion of enrolment for the clinical study to evaluate Imricor’s Vision-MR Ablation Catheter for the treatment of atrial flutter under real-time magnetic resonance imaging (MRI) guidance. The single-centre study enrolled 35 patients and was...
In October 2017, the American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) released a guideline document on the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death (SCD): a guideline document for healthcare professionals....
Vital USA Inc., a healthcare internet-of-things (IoT) vital sign company announced at the Consumer Technology Association's annual meeting (CES; 9-12 January, Las Vegas, USA) the release of the Vital Moto Mod: mobile monitoring platform featuring the first fully integrated platform to measure...
The US Food and Drug Administration (FDA) is providing information and recommendations regarding the Zoll LifeVest 4000 due to concerns that the device may fail to deliver treatment to the patient if the device is not replaced soon after...
The Janssen Pharmaceutical Companies of Johnson & Johnson has announced findings from the US Food and Drug Administration's (FDA) mini-sentinel assessment, confirming the positive safety and efficacy profile of Xarelto (rivaroxaban) established in the phase III ROCKET AF clinical trials,...
Nearly a quarter of patients with chronic ischaemic cardiovascular disease are dead or hospitalised within six months, reports a European Society of Cardiology (ESC) study published in the European Journal of Preventive Cardiology.1
The Chronic Ischaemic Cardiovascular Disease (CICD) pilot registry...
The cardiovascular magnetic resonance (CMR) 2018 meeting organised by European Association of Cardiovascular Imaging and the Society for Cardiovascular Magnetic Resonance will showcase the latest advances in CMR, and how they are improving patient care and outcomes. CMR 2018 will...
iMedrix, a Silicon Valley and Bangalore-based mHealth start up company, has announced the general availability of its product KardioScreen, a CE certified mobile and portable hospital-grade digital electrocardiogram (ECG). The system uses connected technology, bringing a “deep internet-of-things (IoT) architecture...
A study on substernal pacing acute clinical evaluation (SPACE) by Darius Sholevar (Our Lady of Lourdes Medical Center, Camden, USA) et al published in the Heart Rhythm journal has investigated the feasibility of extravascular pacing using a novel substernal electrode...
Abbott has announced the US Food and Drug Administration (FDA) approval for magnetic resonance (MR)-conditional labelling for the Quadra Assura MP cardiac resynchronisation therapy defibrillator (CRT-D) and Fortify Assura implantable cardioverter defibrillator (ICD), two of the company's most widely-used...
NightBalance, a Netherlands-based company developing obstructive sleep apnoea (OSA) therapies, has announced that the Dutch National Health Care Institute (ZIN) issued a positive recommendation to include its sleep apnoea therapy for reimbursement. The recommendation was issued to the Sleep...
This video has been sponsored by Abbott.
The Confirm Rx implantable cardiac monitor (ICM) is the world’s first and only insertable cardiac monitor that combines a quick and minimally invasive procedure with Bluetooth wireless technology, allowing patients to connect using...
A recent study by Lu-Chen Weng (Massachusetts General Hospital, Boston, USA) et al which aimed to quantify and systematically characterise the genetic architecture of atrial fibrillation (AF) has found that genetic variation contributes substantially to AF susceptibility and risk. The...
The Optimizer system developed by Impulse Dynamics has received approval from the China Food and Drug Administration (CFDA). The Optimizer is an implantable cardiac contractility modulation therapy device for chronic heart failure patients. It is the only device therapy...
A retrospective national Swedish registry study by Leif Friberg and Mårten Rosenqvist (Karolinska Institutet, Stockholm, Sweden) has found that the risk of dementia is higher in atrial fibrillation (AF) patients without oral anticoagulant (OAC) treatment. The paper was presented...
The Heart Rhythm Society (HRS), in partnership with WebMD Education, has announced the launch of a free online educational tool for atrial fibrillation (AF). The new interactive AF information tool is part of a larger series of educational activities hosted by WebMD Education to help patients,...
A cluster analysis of the data from the ORBIT-AF observational registry study has been published by Taku Inohara (Duke University Medical Center, Durham, USA) et al in the Journal of the American Medical Association (JAMA). The cluster analysis of...
A small study by Phillip S Cuculich (Washington University School of Medicine, St Louis, USA) et al has shown that radiation therapy, commonly used to treat cancer, can be used as a noninvasive radioablation approach to treat patients with...
Janssen has submitted a supplemental new drug application to the US FDA for two new indications for rivaroxaban (Xarelto, which is marketed by Bayer in Europe): reducing the risk of major cardiovascular events such as cardiac death, myocardial infarction or...
AliveCor has announced the issuance of a US patent which covers the use of data collected from wearable devices as a means of assisting diagnoses of cardiac arrhythmias, including those that may be asymptomatic.
US Patent Number 9,839,363, entitled "Discordance...
Apple has launched the Apple Heart Study app, a first-of-its-kind research study using Apple Watch’s heart rate sensor to collect data on irregular heart rhythms and notify users who may be experiencing atrial fibrillation (AF).
To calculate heart rate and rhythm, the...
Imricor is developing a magnetic resonance (MR) compatible injection catheter and has announced a joint development agreement with MiRTLE Medical to integrate MiRTLE’s MR compatible 12-lead ECG system with Imricor’s Advantage-MR EP Recorder/Stimulator System. This integration, along with Imricor’s MR...
Following a priority review by US Food and Drug Administration (FDA), evolocumab has received FDA approval in the USA. The drug is a PCSK9 inhibitor for reducing risk of myocardial infarction (MI), stroke and coronary revascularisation.
Pharmaceutical company Amgen has...
AliveCor has added another personal electrocardiogram (ECG) technology product to their range, announcing the US Food and Drug Administration (FDA) have cleared the Kardia Band in the USA. The Kardia Band works exclusively with the Apple Watch, providing owners of...
The Journal of the American Medical Association (JAMA) have published new guidelines on diagnostic testing for obstructive sleep apnoea (OSA) in adults. The new guidelines, authored by Babak Mokhlesi and Adam S. Cifu at the University of Chicago, USA, include updated...
NeuroproteXeon has announced the publication of a second finding from a randomised, controlled phase II trial of inhaled xenon and oxygen combined with hypothermia for out-of-hospital cardiac arrest patients. The study, which appeared in the Journal of the American...
EBR Systems has raised US$50 million to conduct the global SOLVE-CRT (stimulation of the left ventricle endocardially) study. SOLVE-CRT is a pivotal clinical trial intended to establish safety and efficacy in support of US Food and Drug Administration (FDA) approval WiSE (wireless stimulation endocardially), a product...
Acute myocardial infarction (MI) and stroke patients without medical insurance in the USA face “devastating” and catastrophic health expenses that can bankrupt them, new research shows. The study by Rohan Khera (University of Texas Southwestern Medical Centre, Dallas, USA)...
LivaNova has entered a binding letter of intent for the sale of its cardiac rhythm management business franchise to Shanghai-based company MicroPort for $190 million in cash. The companies, which have previously worked together in a joint venture, recently...
1.
The "ILL-CONCEIVED" pallas study may be relaunched
John Camm
"I want to say just a few quick words about dronedarone", John Camm (St George's University of London and Imperial College, London, UK) told the audience of his talk at the Europe...
A study published in the Circulation journal has shown that the risk of atrial fibrillation (AF) in young adults and children who suffer from congenital heart disease (CHD) was 22 times higher than in a matched control group.
The study...
A new comparative study between dabigatran and warfarin finds similar ischemic stroke risks, but fewer brain bleeds for dabigatron compared with warfarin in US-based national drug surveillance network. More research is needed however, to determine whether dabigatran is associated...
Boehringer Ingelheim has announced results from two new analyses of the phase III RE-VERSE AD study, which evaluated the safety and efficacy of idarucizumab, marketed in the USA as Praxbind, in reversing the anticoagulant effect of Pradaxa (dabigatran etexilate...
Rivaroxaban vascular dose, 2.5 mg twice daily, plus aspirin 100 mg once daily also significantly (p=0.0003) reduces the risk of the composite outcome of major cardiovascular events, major adverse limb events and major amputation in patients with peripheral artery...
Presenters at the American Heart Association (AHA)’s annual Scientific Sessions (11-15 November 2017; Anaheim, USA) have identified the lack of awareness of automated external defibrillators (AEDs) in Latinos, as well as a general reluctance in bystanders to perform CPR...
Aziyo Biologics has announced the launch of their CanGaroo bio envelope specifically for use with subcutaneous implantable cardioverter-defibrillators (S-ICDs). It is the only cardiac implantable electronic device (CIED) envelope available for use with S-ICDs.
CanGaroo is a natural extracellular matrix (ECM)...
Boston Scientific has announced the final five-year outcomes data from the PREVAIL study during a late-breaking clinical trial session at this year’s Transcatheter Cardiovascular Therapeutics (TCT; 29 October-2 November 2017, Denver, USA. The data, in combination with five-year outcomes...
Recycling or reusing cardiac implantable electronic devices (CIEDs) could bring down costs for low-income or uninsured patients. A small study presented at the 30th Mexican Congress of Cardiology (2-5 November, 2017; Guadalajara, Mexico) has shown that doctors were able...
In Illinois, USA, Jesus Garcia has become the first patient in his state to receive the HeartMate 3 (Abbott/St. Jude Medical) left ventricular assist device (LVAD), a new option for physicians managing advanced heart failure patients in need of...
The American College of Cardiology (ACC), along with the American Heart Association (AHA) and the Heart Rhythm Society (HRS) has published new guidelines for the treatment of patients with ventricular arrhythmias and the prevention of sudden cardiac death. The...
Analytics 4 Life, a digital health company applying artificial intelligence to improve and develop existing care pathways, has announced it will be presenting new clinical data on the company's ongoing Coronary Artery Disease Learning and Algorithm Development (CADLAD) study...
Published in the Journal of the American College of Cardiology (JACC): Clinical Electrophysiology, Björkenheim et al’s study of patient- and physician-reported outcomes of atrial fibrillation (AF) ablation has analysed the complex variables and subjectivity in assessing AF-related symptoms. The...
Nexeon MedSystems has announced its completion of an initial series of clinical studies evaluating the utilization of transcutaneous auricular vagus nerve stimulation (aVNS) for the relief of paroxysmal atrial fibrillation. The company was previously awarded a €3.4M research grant...
Acutus Medical has announced that the US Food and Drug Administration (FDA) cleared the AcQMap high resolution imaging and mapping system as well as the AcQMap 3D imaging and mapping catheter for use in patients for whom electrophysiology procedures...
A Korean nationwide cohort study by Tae-Hoon Kim et al has shown that truly low-risk Asian patients with atrial fibrillation (AF) for stroke can be identified using the CHA2DS2-VASc score. The study was published in the American Heart Association/American Stroke...
This video has been sponsored by LivaNova.
AF is a growing epidemic affecting 33.5 million people today.1 Up to 91% of AF patients have co-morbidities.2 Among these co-morbidities, the guidelines highlight the need to pay attention to sleep apnea.3 Like asymptomatic...
Boston Scientific has announced key data will be presented at next week’s Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 October–2 November, Denver, USA). For example, on 2 November, data for the Watchman left atrial appendage closure device will be featured...
The US Food and Drug Administration (FDA) has approved Abbott St. Jude Medical’s Confirm Rx insertable cardiac monitor (ICM), a smartphone compatible ICM designed to help physicians remotely identify cardiac arrhythmias. The device may provide a new way for patients...
The risk of atrial fibrillation (AF) is increased in subclinical hyperthyroidism, but it has been is uncertain whether variations in thyroid function within the normal range or subclinical hypothyroidism are also associated with AF. A study published in Circulation...
In October 2016, St. Jude Medical recalled a subset of implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators (CRT-Ds) due to reports of rapid battery failure. To address the issue, the company has released a battery performance alert...
Respicardia has received US Food and Drug Administration (FDA) approval of its remedē system, a transvenous implantable neurostimulation device that stimulates the phrenic nerve and engages the diaphragm to restore natural breathing during sleep in patients with central sleep apnoea...
The American College of Cardiology (ACC), Heart Failure Society of America (HFSA), Heart Rhythm Society (HRS) and Society for Cardiovascular Angiography and Interventions (SCAI) have announced a partnership to develop new modules to help subspecialty cardiologists—interventional cardiologists, electrophysiologists, and...
Abott has announced Conformité Européenne (CE) Mark for 3 Tesla (T) magnetic resonance-conditional labelling for both the Assurity MRI pacemaker and the Tendril STS pacing lead. Patients implanted with these low-voltage devices will have the ability to undergo full-body...
A study published in the American Heart Association’s journal Circulation has found that men develop atrial fibrillation (AF) about a decade earlier than women on average, and being overweight is a major risk factor.
Untreated AF increases the risk of heart-related...
A retrospective study conducting a safety assessment and comparison between two percutaneous devices commonly used for left atrial appendage (LAA) closure in the US has shown a significant increase in reported safety events following US FDA approval of the...
According to the results of the EMANATE trial, patients with atrial fibrillation (AF) who receive apixaban (Eliquis, Bristol-Myers Squibb/Pfizer) before undergoing elective cardioversion of patients with atrial fibrillation to normal sinus rhythm have a lower risk of stroke than...
Pamela B Morris has been selected as the next vice chair of the American College of Cardiology (ACC) annual scientific session. She will serve as vice chair for ACC.19 and ACC.20, and will transition to chair for ACC. 21...
Catheter ablation of atrial fibrillation significantly reduces mortality and hospitalisation in atrial fibrillation patients with heart failure in comparison with conventional treatment, data from the CASTLE-AF trial suggest. Investigators Nassir Marrouche (University of Utah Health Care, Salt Lake City,...
Two novel smartphone and tablet applications (apps) for atrial fibrillation patients and healthcare professionals have been launched by heart experts. The two apps will communicate to maximise face-to-face time. The objectives and design of the apps are outlined in...
Closure of the left atrial appendage during open heart surgery protects the brain from infarctions and stroke, and could be routinely performed in the future, according to the findings from a late-breaking trial presented at the European Society of Cardiology...
Highlights:
- Catheter ablation lowers mortality in atrial fibrillation patients with heart failure
- Thrombus formation on LAA occlusion devices strongly associated with higher risk of ischaemic stroke
- Profile: Sabine Ernst
- EHRA: Inventors Award
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2017/10/38-Cardiac-Rhythm-News-EU.pdf
Sabine Ernst (lead electrophysiology research/consultant cardiologist, Royal Brompton and Harefield Hospital, London, UK and associate lead for Cardiac Imaging in the Biomedical Research Unit of the Royal Brompton Hospital) set up the first magnetic navigation catheter ablation laboratory in...
Highlights:
- Catheter ablation lowers mortality in atrial fibrillation patients with heart failure
- Thrombus formation on LAA occlusion devices strongly associated with higher risk of ischaemic stroke
- Profile: Sabine Ernst
- EHRA: Inventors Award
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2017/10/38-Cardiac-Rhythm-News-US.pdf
Peerbridge Health has announced that its first product, the Peerbridge Cor System—a wireless electrocardiogram (ECG) monitor—has received 510(k) clearance from the US Food and Drug Administration (FDA). The patented device has the smallest on-body footprint of any wearable monitor...
Boston Scientific Corporation has announced a definitive agreement to acquire Apama Medical, a privately-held company that is developing the Apama Radiofrequency (RF) Balloon Catheter System for the treatment of atrial fibrillation (AF). The transaction consists of US$175 million in cash up-front and a...
The US Food and Drug Administration (FDA) has approved Medtronic’s HeartWare HVAD system as a destination therapy for patients with advanced heart failure who are not candidates for heart transplants.
Designed to help the heart pump, the left ventricular assist...
A study has shown modified human factor X to be a safe and effective reversal agent for prevention and treatment of bleeding in patients taking factor Xa oral anticoagulants. This new therapeutic factor X was inspired by a snake...
LivaNova and MicroPort’s Shanghai-based joint venture—MicroPort Sorin Cardiac Rhythm Management—has obtained approval for Rega. The family of pacemakers is now approved by the China Food and Drug Administration.
Rega are the first pacemakers manufactured by the venture, as well as...
CardioFocus’ HeartLight Excalibur Balloon has been granted CE mark. The technology is designed for the treatment of atrial fibrillation.
The Excalibur balloon builds on the universal balloon design of the company’s HeartLight endoscopic ablation system. It is intended to optimise...
The American College of Cardiology (ACC) and the American Heart Association (AHA) have released updated clinical performance and quality measures to benchmark and improve the quality of care for adult patients hospitalised with ST-elevation and non–ST-elevation myocardial infarction (STEMI...
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has issued a positive opinion for an update to the European Summary of Product Characteristics (SmPC) of dabigatran etexilate (Pradaxa, Boehringer Ingelheim) for the...
Boston Scientific has launched the Resonate family of implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) systems featuring the HeartLogic Heart Failure Diagnostic to help physicians improve heart failure (HF) management. The new devices, which are approved...
A study led the University of Warwick, Coventry, UK, has suggested that people are reluctant to use public access defibrillators (AEDs) to treat cardiac arrests.
The analysis of existing international studies, which has been published in the European Heart Journal,...
Abbott has received US Food and Drug Administration (FDA) approval for magnetic resonance (MR)-conditional labelling for one of the company’s most widely-used implantable cardioverter defibrillators (ICD) and associated high voltage leads. The approval of MR-conditional labelling for the Ellipse...
The president of the American College of Cardiology (ACC), Mary Norine Walsh, has said that “it is concerning” that the Graham-Cassidy proposal (for US healthcare reform) is “being rushed to the Senate floor not only at the expense of...
The results of CLOSE, Gore Reduce and the extended follow-up of RESPECT—all published in The New England Journal of Medicine—indicate that percutaneous closure of patent foramen ovale (PFO) is associated with a significant reduction in ischaemic stroke in patients...
Pauline Quenin (I’institut du thorax, INSERM, CNRS, UNIV Nantes, CHU Nantes, Nantes, France) and others report in Circulation Arrhythmia and Electrophysiology that a quarter of families who underwent screening after the sudden unexplained death of a young relative were...
A new study has found that beta blockers are not needed after a heart attack if survivors are taking ACE inhibitors and statins. The study is the first to challenge the current clinical guideline that heart-attack survivors should take...
According to a new survey, the average age and income of cardiologists in the US are increasing.
The fifth annual Cardiovascular Provider Compensation & Production Survey from MedAxiom has revealed that one in five cardiologists are over 61 years...
Experts at Queen’s University Belfast, Belfast, UK, have designed a flexible battery that could provide an alternative to the rigid batteries that usually power medical implants.
Currently, devices such as pacemakers, defibrillators and neurostimulators are fitted with rigid and metal...
Boston Scientific has announced new data from the MultiSENSE (Multisensor chronic evaluation in ambulatory heart failure patients) study, which is evaluating the performance of the HeartLogic heart failure diagnostic to predict impending heart failure decompensation.
Results from the study...
The committee for medicinal products for human use (CHMP) of the European Medicines Agency (EMA) has issued a positive opinion for an update to the European summary of product characteristics of dabigatran (Boehringer Ingelheim) for the treatment of patients...
The Heart Rhythm Society (HRS) has issued an international consensus statement that comprehensively addresses lead management for patients with cardiovascular implantable electronic devices (CIEDs).
The expert consensus statement, which was presented at the 10th Asia Pacific Heart Rhythm Society (APHRS)...
Fysicon has announced that it has received 510(k) FDA clearance for its QMAPP haemodynamic monitoring system. A press release reports that QMAPP offers cardiologists the most advanced technology available in haemodynamic monitoring. It adds that QMAPP amplifier has the...
PaceMate has announced the appointment of Kevin Campbell as its new chief executive officer (CEO). A press release reports that Campbell’s work as a leading cardiologist—combined with his past roles as CEO at K-Roc Healthcare Consulting and advisor to...
The latest results from one of the largest, ongoing global disease registries in atrial fibrillation (AF)— GARFIELD-AF (global anticoagulant registry in the field-atrial fibrillation) —show that there are significant differences in the characteristics of patients with AF in Asia...
AtriCure has launched its AtriClip Pro•V left atrial appendage exclusion system in the USA. A press release reports that the new device offers an open-ended design combined with a tip-first closure mechanism to enable easier navigation and placement when...
There is a lack of sufficient data about the effect of racial differences on outcomes following an out-of-hospital cardiac arrest. Therefore, Karuppiah Arunachalam and colleagues—using results from the national inpatient sample (NIS) database—sought to determine what role (if any)...
AliveCor, the leader in FDA-cleared personal ECG technology, have announced the results of four clinical research presentations that demonstrate that AliveCor's hyper-fast, 30 second, digital ECG can positively impact and potentially even save the lives of millions of people...
New subanalysis data demonstrate edoxaban (Lixana, Daiichi Sankyo) provide comparable efficacy and greater safety compared to warfarin, across non-valvular atrial fibrillation patients with different stroke risk scores. The findings are based on a subanalysis of the ENGAGE AF-TIMI 48...
The first health economics data from GARFIELD-AF (Global anticoagulant registry in the field–atrial fibrillation) was presented at the European Society of Cardiology (ESC) congress (26–30 August, Barcelona, Spain). These data demonstrate that atrial fibrillation imposes a high financial, economic...
The COMPASS study, which was presented at the European Society of Cardiology (ESC) congress (26–30 August, Barcelona, Spain), has shown that rivaroxaban (Xarelto, Bayer) 2.5mg twice daily, plus aspirin 100mg once daily, is associated with a 24% relative risk...
At the European Society of Cardiology (ESC) congress (26–30 August, Barcelona, Spain), the European Heart Rhythm Association (EHRA) released an analytical supplement to the 10th EHRA White Book. The EHRA White Book, which is supported by Biotronik, is the annual...
Data from the EMANATE trial, which was presented at a Hot Line session of the European Society of Cardiology (ESC) congress (26–30 August, Barcelona, Spain), indicate that the risk of stroke is significantly lower in atrial fibrillation patients who...
The FDA has approved the Full MagLev HeartMate 3 (Abbott) left ventricular assist device for use in heart failure patients in need of short-term haemodynamic support (eg. bridge-to-transplant or bridge to myocardial recovery). The device, a press release reports,...
Abbott has initiated a US pivotal clinical study evaluating the safety and effectiveness of a modified version of its Amplatzer device to correct patent ductus arteriosus—a common congenital heart defect that occurs in approximately 80,000 preterm infants in the...
The RE-DUAL PCI trial indicates that dual therapy with the non-vitamin K antagonist oral anticoagulant dabigatran (Pradaxa, Boehringer Ingelheim)—and not aspirin—and a P2Y12 inhibitor is associated with a significant reduction in major bleeding, compared with conventional triple therapy, in...
Preliminary results from the ABLATOR observational registry have shown similar success rates (around 58%) in first time ablation and repeat ablation for persistent atrial fibrillation (AF) ablation procedures with contact force sensing technology after one year of follow-up. A...
Jonathan Behar (London, UK) co-inventor for the Guide CRT platform—shortlisted as a 2017 finalist for the EHRA Inventors Award—details the benefits of this novel technology, which enables the real-time analysis and fusion of cardiac magnetic resonance imaging (MRI)-derived scar...
For the second-year running, the European Heart Rhythm Association (EHRA) held the EHRA Inventors Award competition at EHRA-EUROPACE CARDIOSTIM (18‒21 June, Vienna, Austria), a key activity part of the EHRA Innovation Forum which seeks to facilitate innovation in the...
Behzad Pavri (Thomas Jefferson University Hospital, Philadelphia, USA) is an advocate for re-use of cardiac implantable electronic devices (CIEDs) as life-saving medical technology for patients in low-income countries, which in most cases have limited access to them. For the...
Inspired by the 2017 Heart Rhythm Society (HRS) Scientific Sessions opening plenary theme “Becoming a citizen of the world” and the key speaker, Hugh Evans (humanitarian, social entrepreneur and co-founder/chief executive officer at Global Citizen/Global Poverty Project), HRS highlighted...
Medtronic has received CE mark for the Attain Stability Quad MRI SureScan left heart lead. The device offers active-fixation technology, designed for precise lead placement and stability.
The company has undertaken a limited European launch, with the first commercial implants...
The US Food and Drug Administration has approved Biotronik’s Edora HF-T QP magnetic resonance (MR)-conditional cardiac resynchronisation therapy pacemaker (CRT-P). The device—which features MRI AutoDetect technology—has now been commercially launched by the company in the USA.
Outside of the USA,...
New research has discovered a potential means to trigger damaged heart cells to self-heal. For the first time, researchers have identified a long non-coding ribonucleic acid (ncRNA) that regulates genes controlling the ability of heart cells to undergo repair...
ARCA biopharma has announced the completion of enrolment for GENETIC-AF. This Phase 2B, double-blind, superiority clinical trial is evaluating bucindolol hydrochloride (Gencaro) as a potential genetically-targeted treatment for atrial fibrillation (AF). ARCA expects to report top-line Phase 2B data...
Physicians identified a majority of patients with advanced heart failure as at high risk for transplant, left ventricular assist device (LVAD) or death while few of those patients considered themselves to be at high risk, according to a study...
Marijuana use is associated with a three-fold risk of death from hypertension, according to research published in the European Journal of Preventive Cardiology.
“Steps are being taken towards legalisation and decriminalisation of marijuana in the USA, and rates of recreational...
Portola Pharmaceuticals has announced that the US Food and Drug Administration (FDA) has found its resubmitted Biologics License Application andexanet alfa (AndexXa) to be acceptable for review, with an action due date of 2 February 2018.
The resubmission includes supplemental...
Children from socially and economically disadvantaged families and neighborhoods appear more likely to have thicker carotid artery walls. In adults, this may indicate higher risk for heart attack and stroke in later life.
“We know that socioeconomically disadvantaged people are...
The first study to assess the clinical impact of remote monitoring with the Reveal LINQ implantable loop recorder (Medtronic) has demonstrated the benefits of this feature in early diagnosis of asymptomatic but serious arrhythmias in a significant proportion of...
When speaking on therapy of vasovagal syncope (VVS), it is important to stress that a specific treatment of VVS is only rarely necessary in clinical practice. Indeed, in the majority of patients, VVS is a benign condition that does...
By Angela Gonzalez
A retrospective study, including the largest series of patients with refractory ventricular tachycardia undergoing cardiac sympathetic denervation for structural heart disease, has shown freedom from ventricular tachycardia/implantable cardioverter defibrillator (ICD) shock, heart transplant death of 50% at...
Stereotaxis and Princess Grace Hospital in Monaco have announced the publication of a study comparing the speed of lesion formation of magnetic catheters using the Niobe system to manually controlled contact force catheters.
The analysis, published online in the Journal of Cardiovascular Electrophysiology (DOI: 10.1111/jce.13222), included 1,008...
Pneumonia or sepsis in adults that results in hospital admission is associated with a six-fold increased risk of cardiovascular disease in the first year, according to research published in the European Journal of Preventive Cardiology. Cardiovascular risk was more...
CardioFocus has announced that the Japanese Ministry of Health, Labour and Welfare has approved the HeartLight Endoscopic Ablation System for the treatment of paroxysmal atrial fibrillation (AF) in Japan.
The HeartLight System is a visually-guided laser balloon technology for controlled and consistent...
National charity, Heart Research UK has granted funding for an innovative research project with University College London, UK, and Great Ormond Street Hospital, London, UK, which aims to pioneer a new imaging technique that may benefit babies born with...
By Angela Gonzalez
People working 55 hours or more a week had an approximately 40% higher risk of atrial fibrillation compared with people working 35 to 40 hours a week, a large multi-cohort, 10-year study has found.
Nine out of ten...
Physicians in internal medicine and geriatrics and even physicians working in cardiology departments lack basic knowledge of treatment with implantable cardioverter defibrillators (ICDs) and how to clinically manage these patients, according to a stratified questionnaire survey conducted in Sweden.
The...
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has issued a positive opinion on a label update regarding the use of 15mg once-daily of the oral Factor Xa inhibitor rivaroxaban (Xarelto, Bayer)...
The US Food and Drug Administration (FDA) has approved Biotronik’s Intica DX and Intica cardiac resynchronisation therapy (CRT)-DX implantable cardioverter defibrillator (ICD) systems, which are now available for sale.
DX technology is designed to eliminate the need for an atrial...
The European Commission has granted marketing authorisation for sodium-free potassium binder, patiromer (Valtessa; Vifor Fresenius Medical Care Renal Pharma, Vifor UK), for the treatment of hyperkalaemia in adults.
Robert Lewis, consultant nephrologist and chief of Service of the Wessex Kidney...
Gaining even a little weight over time may alter the structure and function of heart muscle, affecting long-term risk of heart failure, according to new research published in Journal of the American Heart Association.
Researchers followed 1,262 adults (average age...
AliveCor has announced a collaboration with Mayo Clinic to develop tools for medical and non-medical personnel to easily screen for long QT syndrome (LQTS) early by combining AliveCor’s artificial intelligence (AI) technology with Mayo’s patented algorithms.
Through this collaboration, new...
A review appearing in the Journal of the American College of Cardiology (JACC) has highlighted Vectorious Medical Technologies, an Israeli company developing the world’s first digital wireless sensory implant for measuring left atrial pressure (LAP; currently available CardioMEMS technology...
For the first time this year, late-breaking clinical trials session were held at the Asian Pacific Society of Cardiology (APSC) Congress, to highlight world-class research coming out of the region.
The ASIAN-HF registry, which was presented at the late-breaking clinical...
The Heart Rhythm Society (HRS) has announced eight US facilities to be awarded funding to support the development of the 2017 AFib Screening and Education initiative. The programme is designed to increase awareness of atrial fibrillation among patients and caregivers, support...
A French multicentre analysis of left atrial appendage (LAA) occlusion for stroke prevention in atrial fibrillation patients has found a 5.3% rate of thrombus formation on left atrial appendage occlusion devices. Authors of the study consider “this is not...
Cardiac Insight, a US developer of wearable medical devices and diagnostic software, has announced that its US Food and Drug Administration (FDA)-approved Cardea Solo electrocardiogram monitoring system is now available for diagnosing atrial fibrillation following cardiac ablation.
According to a...
National Institutes of Health (NIH) funding to conduct cardiac arrest research has dwindled in the last decade, and is a fraction of what the government spends to study other leading causes of death, according to new research in Journal...
Final results from RE-VERSE AD study show that idarucizumab, marketed in the US as Praxbind (Boehringer Ingelheim), was able to immediately reverse the anticoagulant effect of dabigatran etexilate mesylate (Pradaxa, Boehringer Ingelheim) in patients in emergency situations.
The effects were...
Chance M Witt and Christopher J McLeod (Rochester, USA) review current treatment of ventricular arrhythmias with cardiac sympathetic denervation.
What is cardiac sympathetic denervation?
Most electrophysiologists are fully aware of the complex interplay between the autonomic nervous system and cardiac arrhythmias....
The first participants have been enrolled in Medtronic’s STOP AF First clinical trial. The trial will evaluate the safety and effectiveness of performing pulmonary vein isolation (PVI) with the Arctic Front Advance cryoballoon in patients with symptomatic paroxysmal atrial...
Biotronik’s Evity cardiac resynchronisation therapy pacemaker (CRT-P) has been launched in Japan. It is the company’s highest performing CRT-P, with a battery life of almost 10 years.
The device includes a new feature, LV VectorOpt, which is designed to allow...
Cardiologs Technologies SAS has received US Food and Drug Administration (FDA) clearance of its ECG Analysis platform, a cloud-based cardiac monitoring-analysis web service powered by artificial intelligence (AI).
Cardiologs’ platform is designed to aid physicians in screening for atrial fibrillation...
The UK’s Information Commissioner’s Office (ICO) has found that the Royal Free National Health Service (NHS) Foundation Trust (London, UK) did not adhere to the UK’s Data Protection Act when it provided patient details to Google DeepMind.
The Trust, a...
LifeTech, at the 2017 Congenital and Structural Intervention Congress (CSI; 28 June—1 July, Frankfurt, Germany), announced the launch of a three-year global postmarket surveillance study for the LAmbre left atrial appendage (LAA) closure system. A press release reports that...
A sudden catastrophic loss of heart function, or cardiac arrest, occurred significantly less among adults who acquired health insurance via the Affordable Care Act (ACA), according to new research in Journal of the American Heart Association.
In a study of...
Philips and Spectranetics have announced that they have entered into a definitive merger agreement. Pursuant to the agreement, a press release reports, Philips will commence a tender offer to acquire all of the issued and outstanding shares of Spectranetics...
Young adults with a history of asthma are at a greater risk of thickening of the left ventricle according to research published today in JACC: Heart Failure.
The prevalence of asthma has been growing during the past decade, occurring in...
The tenth year of data on cardiac arrhythmia treatment is being launched at EHRA EUROPACE - CARDIOSTIM 2017.
The edition marks the ten year anniversary of the European Heart Rhythm Association (EHRA) White Book, which reports on the current status...
Breast implants may disrupt an electrocardiogram (ECG) and could result in a false heart attack diagnosis, according to research presented at EHRA EUROPACE - CARDIOSTIM 2017.
“Our experience shows that breast implants make it difficult to see the heart with...
A novel smartphone application (app) has been developed that can direct first responders to cardiac arrest victims more than three minutes before the emergency services arrive. Each minute has been shown to increase the chance of survival by 10%.
The...
A European network—the European Sudden Cardiac Arrest network (ESCAPE-NET)—has been created to find sudden cardiac arrest causes and compare treatments. In Europe, around 20% of all deaths are caused by sudden cardiac arrest.
ESCAPE-NET is backed by the European Heart...
The scientific congresss of the European Heart Rhythm Association (EHRA) is to become an annual event from 2018. The next congress will take place 18–20 March 2018 in Barcelona, Spain.
“The yearly EHRA Congress will provide European and worldwide electrophysiologists...
Public Health England (PHE) and the Royal Society for Public Health (RSPH) have published “Everyday Interactions”, a report which aims to support healthcare professionals to record and measure their public health impact.
The report and toolkit were developed in close...
Newly diagnosed asymptomatic atrial fibrillation patients have a higher rate of previous stroke than those with symptoms, according to results from the GLORIA-AF registry presented at EHRA EUROPACE - CARDIOSTIM 2017. The findings highlight the need for screening to...
Breastfeeding may reduce a mother’s risk of having a heart attack or stroke later in life, according to new research published in of the Journal of the American Heart Association.
Previous studies have suggested that mothers get short-term health benefits...
New data showing the use of Medtronic’s cardiac resynchronisation therapy (CRT) devices—with its proprietary AdaptivCRT and EffectiveCRT algorithms—to result in lower healthcare system costs and improved therapy delivery in heart failure patients who also have atrial fibrillation (AF), have...
Pacemakers and other cardiac devices can help solve forensic cases, according to a study presented at EHRA EUROPACE - CARDIOSTIM 2017. Devices revealed the time and cause of death in some cases where autopsy failed to do so.
“In forensic...
Stereotaxis has announced the European launch of the e-Contact module, designed to provide physicians with a simple-to-interpret indicator of catheter tip-to-tissue contact.
The e-Contact module is primarily impedance-based which allows for a direct measurement of the capacity to deliver radiofrequency...
The Sound Doctor, a UK-based provider of health-related film and audio content for patients, has announced a series of 40 films on heart failure.
The films, released in partnership with Guy’s and St Thomas’ NHS Foundation Trust and Kings College...
Happitech, Arrhythmia Alliance and Bug Labs are to announce the launch of the Heart for Heart e-health initiative. This programme asks people to participate in the world’s largest crowdsourced heart health initiative by inviting them to contribute their heart...
Medtronic has announced that its Reactive atrial-based antitachycardia pacing (ATP) therapy slows the progression of atrial fibrillation (AF) in patients with implanted cardiac devices. A real-world analysis of nearly 8,800 patients was presented at EHRA EUROPACE-CARDIOSTIM 2017.
According to a...
Researchers have designed a safer antiplatelet drug based on a snake venom, according to new research in Arteriosclerosis, Thrombosis and Vascular Biology.
Scientists from the National Taiwan University, Taipei City, Taiwan, have designed a drug to interact with the protein...
Highlights:
-Could etripamil nasal spray be a “game-changer” for paroxysmal supraventricular tachycardia?
-Long detection in single-chamber ICDs decreases mortality and unnecessary shocks
-Chance M Witt and Christopher J McLeod: Cardiac sympathetic denervation for treatment of ventricular arrhythmias: Where are we now?
-Advertorial: S-ICD...
Highlights:
-Could etripamil nasal spray be a "game-changer" for paroxysmal supraventricular tachycardia?
-Long detection in single-chamber ICDs decreases mortality and unnecessary shocks
-Chance M Witt and Christopher J McLeod: Cardiac sympathetic denervation for treatment of ventricular arrhythmias: Where are we now?
-Advertorial: S-ICD...
This video has been sponsored by LivaNova.
The RESPOND-CRT clinical trial is a relevant and significant study in the field of cardiac resynchronisation therapy. It has answered an important question finally, that optimisation can work, and that optimisation, if done...
The benefits of the Subcutaneous Implantable Cardioverter Defibrillator (S-ICD, Boston Scientific) over transvenous (TV)-ICD, including lower rates of lead-related complications and lower in-hospital complication rates in some instances, have been demonstrated in three recently published head-to-head studies by Friedman...
It is safe to reimplant a new cardiac device after the removal of an infected one, and repeat infection rates are low, a large multicentre prospective study has shown. The findings also suggest that timing of the reimplantation does...
Biotronik has received CE mark approval for 3 tesla (T) full-body scans with its latest range of magnetic resonance (MR) conditional pacemaker systems, including single- and dual-chamber pacemakers from the Edora/Evity/Enitra series.
Biotronik’s ProMRI pacemaker portfolio was previously approved...
Carlos Morillo (Calgary, Canada) was one of the first physicians to document the potential of ablation of the pulmonary vein region as a strategy to treat atrial fibrillation (AF) experimentally. Born in Bogotá, Colombia, Morillo is currently focusing on...
Sex-specific cardiovascular drug dosages are needed to reduce adverse reactions in women, according to a position paper from the European Society of Cardiology (ESC). The paper, published in the European Heart Journal - Cardiovascular Pharmacotherapy, outlines the differences between...
Writing for Cardiac Rhythm News, Jan Steffel (Zurich, Switzerland) reviews current data and practice regarding safety of insertable cardiac monitors (ICMs) implantation in office settings.
ICMs are finding increasingly varied applications—not only in cardiology for the diagnosis of syncope—but also...
The use of a novel implantable string subcutaneous defibrillator (ISSD, Newpace) that has no can, and has been designed to be placed without the need for a surgical pocket, may allow improved patient compliance and aesthetics, is less invasive,...
A phase 2 trial (NODE-1) of a nasal spray formulation of etripamil (Milestone Pharmaceuticals) shows it is safe and effective at terminating paroxysmal supraventricular tachycardia (PSVT) and has the potential to be a “game-changer” in the treatment of the...
Biotronik has announced the availability of the Edora line of devices, the company's first available pacemaker series featuring the company's magnetic resonance imaging (MRI) AutoDetect technology.
Edora SR-T is the smallest MR-conditional pacemaker with automated MRI detection capability available in...
Highlights:
- Uninterrupted dabigatran outperforms warfarin in atrial fibrillation ablation
- Martin Burke: Artificial intelligence
- Roberto Keegan: The new LAHRS
- Transvenous lead extraction is a safe procedure with low complication rates
- In memoriam: Mark E Josephson
- Thomas Deering: Profile
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2017/06/36-Cardiac-Rhythm-News_low-res-EU.pdf
Highlights:
- Uninterrupted dabigatran outperforms warfarin in atrial fibrillation ablation
- Martin Burke: Artificial intelligence
- Roberto Keegan: The new LAHRS
- Transvenous lead extraction is a safe procedure with low complication rates
- In memoriam: Mark E Josephson
- Thomas Deering: Profile
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2017/06/36-Cardiac-Rhythm-News_low-res-US.pdf
This video has been sponsored by LivaNova.
Over the past two decades there have been several optimisation strategies, whether they are echo-guided or EKG-guided, aimed to improve outcomes in cardiac resynchronisation therapy (CRT) patients. However, none of them have worked...
Left atrial appendage closure with the Watchman device (Boston Scientific) is safe and effective at stroke prevention, with a high implant and sealing success, even in patients that are contraindicated to and not using oral anticoagulants, one-year follow-up of...
Two case reports—from Michael Kwasman, cardiac electrophysiologist, Providence Sacred Heart Medical Center, Spokane, USA, and Rakesh Latchamsetty, cardiac electrophysiologist, University of Michigan Health Center, Ann Arbor, USA—demonstrate the potential of Abbott's EnSite Precision™ Cardiac Mapping System.
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2017/05/CRN-36_Abbott-Case-Reports.pdf
Men seem to have worse chemotherapy-induced cardiomyopathy than women despite receiving similar cancer treatments, according to research presented at EuroCMR 2017.
“Cancer patients are living longer because of improved treatment but the side effects of treatment include cardiovascular morbidity and...
Patients who suffer superior vena cava tears (SVC) during lead extraction are more likely to survive when treatment includes an endovascular occlusion balloon, a late-breaking trial study presented at the Heart Rhythm Society’s 38th Annual Scientific Sessions (10‒13 May,...
Initial real-world experience with the S-ICD (subcutaneous implantable cardioverter defibrillator) in the USA show that implant success rate is high and short-term complications acceptably low.
Michael R Gold, of the Medical University of South Carolina, Charleston, USA, presented the findings...
Unnecessary shocks and all-cause mortality are reduced in patients treated with a single chamber implantable cardioverter defibrillator (ICD) programmed with a long detection window.
Lead investigator Maurizio Gasparini (Humanitas Research Hospital, Rozzano-Milano, Italy) presented the findings from the ADVANCEIII trial...
New data supporting the clinical performance of the company's exclusive EffectivCRT diagnostic and AdaptivCRT algorithm in heart failure patients who receive cardiac resynchronisation therapy (CRT) devices have been presented at Heart Rhythm 2017, the Heart Rhythm Society's 38th Annual...
Postmenopausal women who reached menopause at an earlier age or who never gave birth are at a higher risk for heart failure, according to research published today in the Journal of the American College of Cardiology.
Previous research has found...
Many patients with an irregular heartbeat, known as atrial fibrillation, are not receiving recommended blood thinning medication they need to prevent strokes, according to a study published today in the Journal of the American College of Cardiology.
People who have...
A substantial number of patients at risk for atrial fibrillation (AF) may remain undetected using conventional monitoring techniques, a late-breaking clinical trial session at the Heart Rhythm Society’s 38th Annual Scientific Sessions (10‒13 May, Chicago, USA) heard. Investigators for...
The Micra leadless pacemaker continues to demonstrate a high implant success rate and a low level of major complications at 30 days in a real world setting, and in the hands of operators with no prior experience in Micra...
Siemens Healthineers is joining forces with Imricor Medical Systems to develop an integrated system that combines the clinical benefits of real-time magnetic resonance imaging (MRI) scans with 3D-guided cardiac ablation.
The companies’ goal is the development of MRI-compatible devices that...
Medtronic has received US Food and Drug Administration (FDA) approval for a portfolio of quadripolar cardiac resynchronization therapy-pacemakers (CRT-Ps) that are designed to improve therapy delivery for patients with heart failure. These devices also allow patients to receive magnetic...
When patients with heart failure were re-hospitalised within a month, those who returned to the same hospital were discharged quicker and were more likely to survive, according to new Canadian research in the Journal of the American Heart Association.
In...
The first three persistent atrial fibrillation patients have been treated using Adagio’s cryolinear ablation technology. This follows the successful treatment of over 25 arrhythmia patients in the company's CryoCure clinical trial.
The Adagio cryolinear ablation technology is designed to enable...
Abbott has announced CE mark and first use of the new Confirm Rx ICM. According to a company release, this is the world’s first smartphone compatible ICM that will help physicians identify difficult to detect cardiac arrhythmias, including atrial fibrillation...
The US Food and Drug Administration (FDA) has approved Boston Scientific’s Resonate family of implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy defibrillator (CRT-D) systems.
The approval includes new features in the Resonate devices including SmartCRT technology with Multisite Pacing...
Biotronik has announced US Food and Drug Administration (FDA) approval of the company's MultiPole pacing (MPP) technology, designed to provide physicians with additional treatment options for heart failure patients who have been non-responsive to cardiac resynchronisation therapy (CRT). MPP...
Biotronik has announced the availability of the first US Food and Drug Administration (FDA)-approved cardiac rhythm management (CRM) devices with technology that automatically recognises when a patient enters a magnetic resonance imaging (MRI) environment.
The company’s MRI AutoDetect technology is...
The US Food and Drug Administration (FDA) has approved Biotronik’s Sentus ProMRI, the thinnest quadripolar left ventricular lead available in the country.
Sentus ProMRI is approved for use with heart failure devices based on data collected during the QP ExCELs...
Remote monitoring of implantable cardioverter defibrillators (ICD), particularly in cardiac resynchronisation therapy (CRT-D), reduces direct healthcare costs compared with standard monitoring, an economic evaluation of the results of the EFFECT trial has found.
The EFFECT study was a multicentre observational...
Early post-procedural mortality occurred in 5% of cases of radiofrequency catheter ablation in patients with scar-related ventricular tachycardia (VT), with more than half of the events occurring in hospital, a trial of a contemporary cohort of patients has found.
The...
Boston Scientific has announced the schedule of key data presentations, including two late-breaking clinical trials that will be featured at the Heart Rhythm Society’s 38th Annual Scientific Sessions (10‒12 May, Chicago, USA).
Notably, the post-market approval data collected on the...
Surgical left atrial appendage occlusion (LAAO) is associated with a reduction of thromboembolism and all-cause mortality among older atrial fibrillation (AF) patients undergoing cardiac surgery, according to an observational study in the USA.
The results were presented in a...
Acutus Medical has announced the FDA clearance of the AcQGuide Steerable Sheath, which marks the first US regulatory milestone for the company. The AcQGuide Steerable Sheath is a percutaneous catheter introducer designed to provide additional maneuverability to catheters that are...
Thomas F Deering (Piedmont Heart Institute, Atlanta, USA) considers that the biggest challenge in electrophysiology (EP) is to “move from the volume to the value world of healthcare delivery” finding ways to improve lives in a “quality and cost-efficient...
Teleflex has announced 510(k) clearance from the US Food and Drug Administration (FDA) for its AC3 Optimus Intra-Aortic Balloon Pump (IABP).This device helps a weakened heart pump blood and can deliver IABP therapy to a broad range of patients, even...
Unemployment is associated with a 50% higher risk of death in patients with heart failure, according to research presented at Heart Failure 2017 and the 4th World Congress on Acute Heart Failure.1 The observational study in more than 20...
Death in patients with heart failure is inversely related to the wealth of the country they live in, according to late breaking results from the INTERCHF study presented at Heart Failure 2017 and the 4th World Congress on Acute...
A new AF atrial fibrillation (AF) patient survey carried out by the AF Association has been launched. Results showed that 79% of patients surveyed—who are currently on a vitamin K antagonist such as warfarin—said that they would favour the...
Physical activity can lower the risk of heart damage in middle-aged and older adults and reduce the levels of heart damage in people who are obese, according to research published today in JACC: Heart Failure.
Obesity is associated with structural...
Mark E Josephson, a pioneer of clinical cardiac electrophysiology (EP), passed away at the age of 73 on 11 January 2017 after a courageous battle with cancer. With his passing, the EP community lost a great physician whose achievements...
At the last Latin American Society of Electrophysiology and Cardiac Stimulation (SOLAECE) Congress (Porto Alegre, Brazil, November 2016) the Executive Board of SOLAECE announced a new proposal to strengthen the positioning of the society into the global context of...
Boston Scientific has initiated a worldwide study (MADIT S-ICD) that will evaluate the survival benefit of patients treated with the Emblem MRI Subcutaneous Implantable Defibrillator (S-ICD) System who are aged 65 and older with a history of prior heart...
Working-age people who have syncope have a higher risk of occupational accidents and job loss, compared to adults without the condition, according to new research in Circulation: Cardiovascular Quality and Outcomes.
In a Danish study comparing adults age 18 to 64 with recurrent...
Patients with refractory reflex vasovagal syncope who received a pacemaker programmed with Closed Loop Stimulation (DDD-CLS, algorithm from Biotronik) had a seven-fold reduction in fainting compared with patients in a placebo pacing group, according to results from the SPAIN...
Biotronik has announced the European launch of a new lead for tachycardia therapy that features a helical design for increased long-term performance through stress reduction. The Plexa ProMRI lead is made for use with implantable cardiac defibrillators (ICDs) as...
Women who are taller, heaver and have a greater body surface area have a nearly threefold greater risk of atrial fibrillation than smaller women, according to research presented at EuroPrevent 2017 (6-8 April; Malaga, Spain). The study included 1.5...
Medtronic’s ENDURANCE Supplemental trial of the company’s HVAD ventricular assist device has failed to meet its primary endpoint of all neurological events at 12 months. However, secondary results have shown superior results for patients treated with the system than...
Janssen Pharmaceuticals has collaborated with Premier on the first and largest study of its kind to address an unmet medical need for hospitalised patients with atrial fibrillation (AF) who are at risk for ischaemic stroke.
Named QUANTUM AF (Quantify...
Medtronic has enrolled the first participants in the STOP Persistent AF clinical trial. The trial will evaluate the safety and effectiveness of a pulmonary vein isolation-only strategy for treating patients with persistent atrial fibrillation (AF), using the Arctic Front...
The first patient has been enrolled into Daiichi Sankyo’s ELIMINATE-AF study. The multinational, randomised phase 3b study will explore the safety and efficacy of the company's oral, once-daily direct factor Xa-inhibitor edoxaban (Lixiana/Savaysa) against a vitamin K antagonist in...
The implantable cardioverter defibrillator (ICD) is a well-established therapy for the prevention of sudden cardiac death. However, it is still associated with complications over time to the patient, leading to re-operation rates of 15.5% at six years.1 Many of...
Cardiac lead extraction is safer in high volume centres, according to the largest study of contemporary practice in Europe, published in European Heart Journal. Extraction in a low volume centre was associated with a doubled risk of death while...
Biosense Webster has launched of the Carto Visitag module with Ablation Index, a new technology providing visual indication based on the integration of power, contact force and time parameters to be displayed on the Carto 3 system.
The index...
Aegis Medical Innovations has received Investigational Device Exemption approval from the US FDA to initiate a clinical trial in the US for its Sierra ligation system. Aegis developed the Sierra technology in partnership with the Mayo Clinic in Rochester,...
Biotronik has launched the Edora range of high-functioning, small-size pacemakers and cardiac resynchronisation therapy pacemakers (CRT-Ps).
The new devices are equipped with features designed to improve patient safety, therapy and comfort. Moreover, Edora pacemakers are significantly smaller and lighter than preceding...
Automated external defibrillators (AEDs) are associated with increased survival of sudden cardiac arrest when installed in schools, yet only 17 out of 50 US states require AED installation in at least some of their schools, according to an analysis...
Results from the RE-CIRCUIT study have shown that uninterrupted dabigatran (Pradaxa, Boehringer Ingelheim), was associated with fewer bleeding complications than uninterrupted warfarin before, during and after atrial fibrillation (AF) treatment with ablation.
The findings offer evidence that dabigatran is a...
The National Institute for Health and Care Excellence (NICE) has issued medical technology guidance recommending the use of Boston Scientific’s cardiac resynchronisation therapy defibrillators (CRT-D) powered by EnduraLife battery technology for treating patients with heart failure.
In their evaluation, NICE—which...
Mary Norine Walsh has become president of the American College of Cardiology during the Convocation Ceremony held in conjunction with the society’s 66th Annual Scientific Session (ACC; 17-19 March, Washington, DC, USA).
Walsh is the director of the heart failure...
For patients with persistent atrial fibrillation or those who are at high risk for recurring atrial fibrillation, catheter ablation is recommended, followed most often by continued use of blood thinners, regardless of whether the ablation procedure was effective. However,...
Researchers at Cleveland Clinic, Cleveland, USA, put five popular wrist-worn fitness trackers to the test to find out how accurately they gauge heart rate across several types of exercise and intensity levels.
Based on their findings, the old-fashioned chest strap...
AliveCor has release the Kardia Pro, an industry-first artificial intelligence-enabled platform for doctors to monitor patients for the early detection of atrial fibrillation, in the USA.
The company has also announced US$30 million in new funding, led by Omron Healthcare...
Community coffee shops and cash machines might be ideal locations for public access to automated external defibrillators, according to new research published in Circulation.
An automated external defibrillator is a computerised medical device that can check a person’s heart rhythm...
As the Zika virus continues to spread globally, new evidence has emerged about the virus’s potentially detrimental effects on the heart, according to data presented at the 2017 scientific session of the American College of Cardiology (ACC; 17‒19 March, Washington, DC,...
A study outside the clinical trial setting, which included US Medicare patients, has shown the effectiveness of the CardioMEMS HF system (Abbott) to significantly reduce heart failure hospital admissions and improve the quality of life in patients with chronic...
Newly published findings suggest that certain urinary biomarkers could help clinicians identify risk of acute kidney injury in patients with acute decompensated heart failure. The study, published in Clinical Cardiology, is the first to investigate the risk assessment capabilities...
Patients with heart failure in the USA are more likely to be hospitalised and more likely to die during the colder winter months, according to two studies presented at the American College of Cardiology’s 66th Annual Scientific Session (ACC;...
An Italian prospective multicentre registry with a large study population has demonstrated the safety and efficacy of a new hand-powered bidirectional rotational mechanical lead extraction sheath used in chronically implanted leads for more than a year.
The study, led by...
Stereotaxis has reported results of a study conducted at Centre Hospitalier Universitaire (CHU) of Saint-Étienne, France, which validates the advantages of the Niobe ES magnetic navigation system over the Niobe II system in terms of procedure and fluoroscopy times for atrial fibrillation (AF)...
According to a study published in European Heart Journal–Cardiovascular Pharmacotherapy, painkillers that are traditionally considered harmless by the general public are associated with increased risk of cardiac arrest. The authors Gunnar H Gislason (Copenhagen University Hospital Gentofte, Denmark) and...
AtriCure has announced David B DeLurgio as the US principal investigator for the CONVERGE IDE clinical trial.
DeLurgio is a cardiac electrophysiologist at Emory Healthcare in Atlanta, USA. He is director of Electrophysiology at the Emory Heart and Vascular Center at Emory St Joseph’s Hospital, a role...
The American College of Cardiology (ACC), the American Heart Association (AHA) and the Heart Rhythm Society (HRS) have recently released the first US guideline on the evaluation and management of patients with syncope.
The 2017 ACC/AHA/HRS Guideline for the...
The US Food and Drug Administration (FDA) has given 510(k) clearance to Medtronic for its Reveal LINQ Insertable Cardiac Monitor (ICM) with TruRhythm Detection.
A company release states that the Reveal LINQ ICM with TruRhythm Detection offers exclusive algorithms that...
Medtronic has announced that the US Centers for Medicare & Medicaid Services (CMS) has approved coverage for the Micra Transcatheter Pacing System (TPS). This decision, effective immediately, follows the approval of two studies required to enable reimbursement through Medicare's...
Rules governing the conduct of clinical trials are failing to produce the intended benefits for patients and should be rewritten through a transparent process that involves academic clinical trialists and patient advocates as well as regulators and industry representatives,...
The first patient has been enrolled into Daiichi Sankyo’s ENTRUST-AF PCI study. The multinational, randomised phase 3b study will evaluate a treatment regimen based on the company's oral, once-daily direct factor Xa-inhibitor edoxaban (Lixiana/Savaysa) against a vitamin K antagonist-based...
How are smartphones and computer programs transforming healthcare, especially when it comes to preventing, diagnosing and treating heart disease? This is the focus of a collection of articles recently published in the European Heart Journal.
Called eHealth, the topic includes...
While lifestyle patterns, including physical activity and body mass index (BMI), are associated with overall heart failure risk, they are more strongly associated with the heart failure subtype HFpEF, according to a study published in the Journal of the...
Following current dietary recommendations may lead to small improvements in overall heart health in overweight individuals, according to a study published in the Journal of the American College of Cardiology.
The most recent recommendations of the US Dietary Guidelines...
Physicians should be well-versed in the herbal medications heart disease patients may take to be able to effectively discuss their clinical implications, potential benefits and side effects—despite a lack of scientific evidence to support their use, according to a...
Anders Hove has joined Arca Biopharma’s board of directors.
“Anders brings decades of experience building and advising biopharmaceutical companies,” says Michael R Bristow, president and chief executive officer of ARCA. “With his expertise on both the corporate and investment sides of...
Phrenic nerve injury is a well-established complication of all types of atrial fibrillation (AF) ablation and is most common with balloon-based approaches, writes Hugh Calkins (Baltimore, USA) for Cardiac Rhythm News. However, he notes, “with careful monitoring for phrenic...
Results from a large multicentre observational study have shown that patients undergoing cardiac resynchronisation therapy (CRT) upgrade had worse clinical response and long-term survival compared with de novo implantations.
“The number of upgrade procedures from single- or dual-chamber devices to...
The FlexAbility Ablation Catheter, Sensor Enabled is designed to improve the versatility and precision during cardiac ablation procedures to treat atrial flutter.
The Sensor Enabled tool complements the EnSite Precision cardiac mapping system and expands Abbott’s cardiac mapping and ablation...
Electric and magnetic fields generated from everyday household appliances, electrical tools and more, used in very close proximity to the body, can interfere with the ability of pacemakers to regulate patients’ heart rhythm, according to new research published in Circulation.
“Electromagnetic interferences...
The US Food and Drug Administration (FDA) has approved Medtronic’s Freezor Xtra cryoablation catheter for treating patients with atrioventricular nodal re-entrant tachycardia (AVNRT). The Freezor Xtra catheter is a flexible, single-use device used to freeze cardiac tissue and block...
This webinar has been sponsored by Medtronic.
Watch the recent webinar on persistent Atrial Fibrillation (AF) ablation with Prof. Schilling (St. Barts, London), Prof. de Asmundis (Brussels) and PD Dr. Chun (Frankfurt a.M.) and learn about the latest evidence, indications,...
The first-in-man study exploring the safety, feasibility and neurological outcome with the Occlutech left atrial appendage (LAA) occluder (Occlutech) has shown a 93% procedural success rate and acceptable efficacy at three months for stroke prevention in patients with atrial...
The arrhythmogenicity of the superior vena cava has been rarely detected in patients with long-standing persistent atrial fibrillation, according to study from China recently published in Europace.
The superior vena cava, one of the most common non-pulmonary vein foci, “has...
The German Institute for the Hospital Remuneration System (InEK) has renewed Status 1—the highest of four levels—for SentreHEART’s Lariat and Eclipse left atrial appendage closure devices across 50 hospitals under the NUB innovation programme.
This decision allows approved hospitals in...
The phase three COMPASS trial, evaluating the efficacy and safety of rivaroxaban (Xarelto, Janssen) for the prevention of major adverse cardiac events, including cardiovascular death, myocardial infarction and stroke in patients with coronary artery disease or with peripheral artery...
The Texas Cardiac Arrhythmia Institute at St David's Medical Center, Austin, USA became the first facility in the US state of Texas to implant the new Amplatzer Amulet left atrial appendage (LAA) occluder from St Jude Medical to seal...
Boston Scientific has launched the Resonate cardiac resynchronisation therapy defibrillator (CRT-D) systems in Europe. This new family of devices features SmartCRT technology, which recently received the CE mark. The platform is now available for heart failure patients across Europe.
The...
A study published in JAMA Cardiology has shown a link between habitual e-cigarette use and increased cardiac sympathetic activity and oxidative stress.
The 2016 European guidelines on cardiovascular disease prevention flagged up the need for further research on the long-term...
Electrophysiologists Manuel Ayala Patete, Dulce Garcia, Mikel Liñero, Vanesa Burgos and Carlos Garcia Lithgow, all from Centro Cardio-Neuro-Oftalmologico y Transplante (CECANOT) in Santo Domingo, Dominican Republic, write about their experience using the ColumbusTM 3D EP Navigation System (MicroPort) at...
Parents of children with “critical” congenital heart defects – which require at least one cardiac surgery – are at high risk for mental health problems, particularly post-traumatic stress disorder (PTSD), anxiety and depression, according to research in Journal of...
Results from the first randomised study comparing multielectrode mapping vs. conventional point-by-point mapping for ventricular tachycardia (VT) substrate ablation procedures have shown that a multielectrode mapping strategy allows better discrimination of local abnormal electrograms and conducting channels, which may...
Flu and pneumonia vaccines may improve the quality of life and outcomes of heart failure patients by providing cost-effective protection against life-threatening respiratory infections, according to a review paper published today in JACC: Heart Failure.
In a 2005 report, the...
Abbott has announced US Food and Drug Administration (FDA) approval for magnetic resonance (MR)-conditional labelling for both the Assurity MRI pacemaker and the Tendril MRI pacing lead.
Patients implanted with these low-voltage devices will have the ability to undergo full...
Biotronik has announced the launch of a new series of cardiac resynchronisation therapy (CRT) devices with several functionalities that allow physicians to offer an all-inclusive and individualised solution for optimised CRT.
“Biotronik’s new CRT-Ds are equipped with numerous features that...
Medtronic has received US Food and Drug Administration (FDA) 510(k) clearance for the CardioInsight noninvasive 3D mapping system. The CardioInsight system is used to map a wide range of irregular heart rhythms in the upper and lower chambers of...
Heart disease patients taking PCSK9 inhibitors to achieve very low levels of cholesterol do not experience an increase in adverse events, including memory impairment or nervous system disorders, but may have an increased risk of cataracts, according to a...
The US Centers for Medicare and Medicaid Services (CMS) have made the decision to cover leadless cardiac pacemakers, as outlined in the agency's final National Coverage Determination (NCD). This decision will provide Medicare patient access to leadless pacemakers, consistent...
For the tenth year in a row, Biotronik is hosting the “Expert Meeting Berlin” (EMB). Led by Christopher Piorkowski, Heart Center Dresden, Germany, the platform allows cardiologists and electrophysiologists to discuss challenges and opportunities to individualise cardiac rhythm management...
The number of adults living with heart failure increased from about 5.7 million (2009-2012) to about 6.5 million (2011-2014), according to the American Heart Association (AHA)’s 2017 Heart Disease and Stroke Statistics Update.
Based on the latest statistics, the number...
A growing body of evidence suggests that the underlying cause for many cryptogenic strokes is atrial fibrillation (AF). However, many of these patients do not receive additional cardiac monitoring after an initial stroke work up, leaving them at risk...
This educational supplement is only available in countries outside the USA.
This educational supplement contains highlights of the 5th edition of The Future of Heart Failure meeting (30 September‒1 October 2016, Barcelona, Spain), an educational event organised by St. Jude...
CardioFocus has announced the initial clinical evaluation of the HeartLight Excalibur balloon, a next-generation technology designed for the treatment of atrial fibrillation. The device is undergoing an initial clinical evaluation in Europe as part of a broader development programme...
In this case report, Timothy Betts (consultant cardiologist and electrophysiologist, Oxford University Hospitals NHS Foundation Trust, Oxford, UK) details the benefits of the WiSE CRT technology (EBR Systems) to treat heart failure.
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2017/07/CRN-35-EBR-case-report-.pdf
Highlights:
-Case strengthens for opportunistic atrial fibrillation screening
-Multisensor algorithm provides timely alert to predict early sings of worsening heart failure
-Pier Lambiase: Non-invasive imaging
-Advertorial: OSYPKA launches HAT500® RF ablation generator
-Profile: Seth J Worley
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2017/01/35-Cardiac-Rhythm-News_low-res.pdf
Robert Califf has stepped down as US Food and Drug Administration (FDA) commissioner upon the inauguration of Donald Trump as US President on 20 January 2017. Democrat Califf, who was appointed to the post in September 2016 by Barack...
Results of AF-FICIENT, the first-in-man study which seeks to evaluate the safety and performance of the new Apama radiofrequency (RF) balloon catheter system (Apama Medical), have shown the device is safe and allows successful pulmonary vein isolation for paroxysmal...
Intracardiac echocardiograpy (ICE) facilitates a high success implant rate of the Amplatzer Amulet left atrial appendage (LAA) occluder (St Jude Medical/Abbott) as well as a high LAA closure rate to prevent thromboembolism in non-valvular atrial fibrillation (AF), according to...
Biotronik is launching a new series of Edora single- and dual-chamber pacemakers in Japan ahead of its global launch. These devices feature both the company’s ProMRI and Biotronik Home Monitoring technology.
“The size reduction achieved with the latest Biotronik pacemakers...
Of patients over age 65 who received an implantable cardioverter-defibrillator (ICD) after surviving sudden cardiac arrest or a near-fatal arrhythmia, almost 80% survived two years—a higher rate than found in past trials performed to demonstrate the efficacy of the...
Seth J Worley (senior consultant, Cardiac Rhythm Device Management, MedStar Heart & Vascular Institute at MedStar Washington Hospital Center, Washington, DC, USA) considers that cardiac resynchronisation therapy (CRT) is one of the most important developments in the cardiac rhythm...
St Jude Medical has announced that it will immediately deploy the latest release of cyber security updates for its Merlin remote monitoring system, used with implantable pacemakers and defibrillator devices.
According to a company release, the improvements include security updates...
Portola Pharmaceuticals has signed a US$50 million loan agreement with Bristol-Myers Squibb and Pfizer that provides additional funding toward development and clinical studies of andexanet alfa (AndexXa), an investigational compound that is a potential antidote for Factor Xa inhibitors. ...
The Women in Cardiology (WIC) section of the American College of Cardiology (ACC) report that 47% of female cardiologists have avoided being pregnant during periods when they would be exposed to radiation, but 57% have been exposed to radiation...
Abbott has now completed the acquisition of St Jude Medical. The Abbott press release announcing the completion of the acquisition said, “The transaction provides Abbott with expanded opportunities for future growth and is an important part of the company’s...
We are standing at a crossroads of non-invasive imaging technologies in the field of electrophysiology. Advances in cardiac magnetic resonance imaging (CMRI) mean that it is now possible to delineate scars utilising gadolinium late enhancement and T1 mapping; writes...
By Angela Gonzalez
In the quest to reduce the number of atrial fibrillation (AF) related strokes, which account for a third of all ischaemic strokes, there has been a greater interest in recent years in the need to identify unknown...
From the start of this year, American Heart Association (AHA) is offering advanced accreditation for hospitals that meet the high standards needed for treating the most complex cases of heart disease. The Cardiovascular Center of Excellence designation is part...
Cardiologists are highly satisfied in their careers; however, disparities remain between the career experiences of men and women, according to the American College of Cardiology’s third Professional Life Survey published in the Journal of the American College of Cardiology....
Alcohol abuse increases the risk of atrial fibrillation, heart attack and congestive heart failure as much as other well-established risk factors such as high blood pressure, diabetes, smoking and obesity, according to a study recently published in the Journal...
Biotrace Medical's Tempo leadBioTrace Medical has announced the first commercial use of the company’s Tempo temporary pacing lead since US Food and Drug Administration (FDA) 510(k) clearance in October 2016.
The first cases involved patients undergoing transcatheter aortic valve implantation...
The Society of Thoracic Surgeons (STS) has issued new clinical guidelines that include major recommendations for the use of surgical ablation when treating atrial fibrillation. The guidelines, posted online in The Annals of Thoracic Surgery, will appear in the January...
The US Food and Drug Administration (FDA) has issued a final rule to ban powdered surgical gloves, patient examination gloves and the absorbable powder used to lubricate surgical gloves.
Citing associations with serious adverse events—including allergic reactions, lung and airway...
The UK’s National Institute for Health and Care Excellence (NICE) has announced its participation in the US Food and Drug Administration (FDA)’s Payer Communication Taskforce. This programme aims to accelerate US patient access to new technologies by gathering evidence...
St Jude Medical has announced the US Food and Drug Administration (FDA) clearance of its EnSite Precision cardiac mapping system and Advisor FL circular mapping catheter, Sensor Enabled.
The new EnSite Precision cardiac mapping system is the latest addition to...
Join atrial fibrillation (AF) experts Prof Richard Schilling (St Barts Hospital, London UK), Prof Carlo de Asmundis (Universitair Ziekenhuis, Brussels, Belgium) and PD Dr KR Julian Chun (Cardioangiologisches Centrum Bethanien, Frankfurt, Germany) in an interactive webinar aimed to discuss...
Twelve-month results of the REDUCE LAP HF (Reduce elevated left atrial pressure in patients with heart failure) trial have shown both continued device patency and sustained clinical benefits at one year following implantation of a transcatheter atrial shunt.
The...
Christian Butter (Heart Center Brandenburg, Bernau bei Berlin, Germany) was awarded best abstract at the XVII International Symposium on Progress in Clinical Pacing (29 November‒2 December, Rome, Italy) for his presentation of the final outcomes of the SELECT-LV clinical...
People who binge drink often experience an irregular heartbeat or a heart “flutter,” sometimes referred to as “holiday heart syndrome.” However, people who drink smaller amounts of alcohol on a regular basis are also at higher risk of irregular...
Medtronic’s HVAD system left ventricular assist device (LVAD) has received CE mark for a less-invasive implant procedure in patients with advanced heart failure. The company announced the HVAD system is the first centrifugal LVAD to be approved in the...
Corsens Medical has received clearance for a pre-marketing notification (510(k)) with the US Food and Drug Administration (FDA) for its cardiac monitor.
The company received the following indications statement for the Corsens Cardiac Monitor: "The Corsens Cardiac Monitor records vibrational...
Amsel Medical Corporation is to present initial animal results of its “A Novel Mechanical Closure Device for Left Atrial Appendage Orifice and Closure of Right Atrial Incision” research at the upcoming 2016 International Conference for Innovations in Cardiovascular Systems...
This case report has been written by Timothy Betts (consultant cardiologist and electrophysiologist, Oxford University Hospitals NHS Foundation Trust, Oxford, UK) and has been sponsored by EBR Systems.
Conventional cardiac resynchronisation therapy (CRT) is a widely accepted treatment option for patients with...
A proof-of-concept study has demonstrated that a new cryoablation system, which achieves “much cooler” temperatures than previous cryoablation technologies, is able to create large, transmural ventricular lesions from both the endocardium and the epicardium. The results of the study,...
Medtronic has received US Food and Drug Administration (FDA) approval for the Claria MRI Quad cardiac resynchronisation therapy defibrillator (CRT-D) SureScan device for patients with heart failure.
The Claria MRI CRT-D is approved for scanning in both 1.5 and 3...
Piotr Ponikowski (Centre for Heart Disease, Clinical Military Hospital, Wroclaw, Poland), chairperson of the 2016 European Society of Cardiology (ESC) Guidelines for the diagnosis and treatment of acute and chronic heart failure, discussed at the 5th Edition of The...
A prespecified primary analysis of the MOMENTUM 3 US IDE clinical study has indicated improvement in clinical outcomes for advanced heart failure patients implanted with a fully magnetically levitated centrifugal-flow pump (HeartMate 3 left ventricular assist system , St...
Results from the MultiSENSE study have demonstrated that a new multisensor algorithm, the HeartLogic Heart Failure Diagnostic Service (Boston Scientific), provides a timely alert to predict impending heart failure decompensation.
Data were presented by principal investigator John P Boehmer (medical...
In light of the recently presented results from REM-HF and MORE-CARE trials, Gerhard Hindricks and Nikolaos Dagres, both from Leipzig University Heart Center, Leipzig, Germany, share their views on the value of remote monitoring.
At the European Society of...
With the aim to help tackling the limitations of detecting atrial fibrillation (AF) across the globe with a simple pulse check and to raise awareness of the importance of treating the condition at the right time and with the...
Two drug regimens involving low doses of rivaroxaban (Xarelto, Bayer)—one with a 15mg once daily dose of the drug and a P2Y12 inhibitor and another with a 2.5mg twice daily dose and dual antiplatelet therapy—are associated with lower rates...
OSYPKA AG has announced the launch of the HAT500® radiofrequency (RF) ablation system in Europe. The device received CE mark in October 2016.
OSYPKA AG, inventor of the world’s first radiofrequency ablation generator in 1986, presents now its eight generation...
The American Heart Association (AHA) awarded its Basic Research Prize for 2016 to Elizabeth M McNally (Chicago, USA) for “Ground-breaking investigations of novel genetic mechanisms responsible for inherited human disorders including heart failure, cardiomyopathy, muscular dystrophy, arrhythmias and aortic...
Patient enrolment into the international Phase IIIb RE-DUAL PCI study is complete. The study is evaluating the safety and efficacy of dabigatran (Pradaxa, Boehringer Ingelheim) in patients with atrial fibrillation undergoing percutaneous coronary intervention (PCI). A press release notes...
More than a third of advanced heart failure patients treated with a left ventricular assist device (LVAD) and intensive drug therapy have recovered their heart function enough to allow removal of the LVAD device, according to preliminary results of the ongoing RESTAGE...
Health Canada has approved edoxaban (Lixiana, Servier Canada) for the prevention of stroke and for systemic embolic events in patients with non-valvular atrial fibrillation, in whom anticoagulation is appropriate and for treatment of venous thromboembolism (deep vein thrombosis, pulmonary...
Biotronik has announced the US launch of Inventra HF-T, an implantable cardioverter defibrillator (ICD) that delivers “ultra-high” energy with 42 joules (J) on the first shock.
“For an increasing number of patients—specifically those with larger cardiac anatomy and lower ejection...
St Jude Medical has announced CE mark approval for magnetic resonance (MR) conditional labeling for the company’s Quadra Allure MP cardiac resynchronisation therapy pacemaker (CRT-P).
The Quadra Allure MP CRT-P, with MultiPoint pacing technology, offers patients the flexibility to undergo full-body...
A first-in-human study has shown that a new temporary lead for cardiac pacing (Tempo Lead, BioTrace Medical) is safe and effective for use in procedures in which temporary pacing is indicated such as transcatheter aortic valve implantation (TAVI), balloon...
Arrhythmia Alliance, the UK-based heart rhythm charity, is calling on NHS England to reconsider a programme that it says will mean the “hundreds” of patients with atrial fibrillation in England who cannot take oral anticoagulation will be left without...
Martin Burke (CorVita Science Foundation, Chicago, USA) presents the preclinical acute and chronic performance of the combined implant of a leadless anti-tachycardia pacemaker and subcutaneous implantable cardioverter defibrillator (S-ICD). The results have shown appropriate VVI functionality, successful wireless device-device...
Dhiraj Gupta (Liverpool Heart and Chest Hospital, Liverpool, UK) discusses NHS England's commissioning through evaluation for left atrial appendage occlusion (LAAO). This programme is designed to enable a limited number of patients to receive LAAO, a procedure that is...
A study presented at the last late-breaking trial session of the 2016 Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 October-2 November, Washington, DC, USA) indicates that the Amplatzer Amulet left atrial appendage occluder device (St Jude Medical), for the prevention...
Final results from the RESPECT trial found that percutaneously closing a patent foramen ovale (PFO) using the Amplatzer PFO Occluder (St Jude Medical) was superior to medical management in the prevention of recurrent ischaemic stroke in patients who previously...
AtriCure has announced that it has received approval from the Japanese Ministry of Health, Labor and Welfare (MHLW) for its cryoICE platform and CRYO2 cryoablation probe. The devices are used for surgical treatment of cardiac arrhythmias.
“We are pleased to have this...
The FDA has approved St Jude Medical’s Amplatzer patent foramen ovale (PFO) occluder device for the prevention of stroke. Announced on 28 October, this clearance precedes a first report investigation of the final long-term outcomes of the RESPECT trial...
AliveCor has announced the availability of Kardia Band—the world’s smallest single lead medical grade ECG. Kardia Band works with Apple Watch to provide a single lead medical-grade ECG of the user’s heart rhythm to identify possible atrial fibrillation. It...
Acutus Medical has announced the completion of the first patient procedure in the UNCOVER-AF (Utilising novel dipole density capabilities to objectively visualise the etiology of rhythms in atrial fibrillation) clinical study. The study is evaluating the incidence of device and...
BioTrace Medical has received FDA 510(k) clearance for its Tempo Lead, which a press release describes as an innovative temporary pacing lead that has been designed for use in procedures in which temporary pacing is indicated, including transcatheter aortic...
CardioFocus has announced the appointment of Omari Bouknight as chief commercial officer and Jeff Rynbrandt as vice president of US Sales. Together they will lead the commercialisation of the HeartLight Endoscopic Ablation System, which was recently FDA-approved for paroxysmal atrial fibrillation (AF), in...
Highlights:
-Rethink of ICD implantation in non-ischaemic heart failure may be on the cards
-Remote monitoring for heart failure may not improve clinical outcomes
-Stefan Spitzer: The OASIS trial: A critical appraisal
-Mohammad Shenasa: Relevance of imaging for ventricular tachycardia ablation
-Advertorial: WiSE technology...
Jeremy Ruskin (Boston, USA), professor of medicine Harvard Medical School and director emeritus of the Cardiac Arrhythmia Service and Clinical Electrophysiology Laboratory at Mass General Hospital (MGH), has mentored and trained over 120 clinical and research fellows over the...
Tobias Brügmann (Institute of Physiology I, University of Bonn, Life and Brain Center, Bonn, Germany) and others report in The Journal of Clinical Investigation that optogenetic defibrillation— using expression of the light-sensitive-channel channelrhodopsin-2 (ChR2) in cardiac tissue—terminated ventricular arrhythmias...
Cardiac Rhythm News speaks to Richard Schilling (St Bartholomews & The Royal London Hospital, London, UK), president-elect of BHRS (2017‒2019), about his objectives during his tenure, the challenges ahead in the cardiac rhythm field in the United Kingdom and...
A first-of-its-kind study has found that male veteran athletes have a significantly higher prevalence of arrhythmias such as atrial fibrillation, atrial flutter, sinus pauses, and “alarmingly” non-sustained ventricular tachycardia when compared to sedentary controls, according to research presented at...
A first-of-its-kind study has found that male veteran athletes have a significantly higher prevalence of arrhythmias such as atrial fibrillation, atrial flutter, sinus pauses, and “alarmingly” non-sustained ventricular tachycardia when compared to sedentary controls, according to research presented at...
Stereotaxis has been awarded a patent protecting its Vdrive robotic navigation system with V-Loop variable loop catheter manipulator by the State Intellectual Property Office of the People’s Republic of China.
The patent is the second awarded to Stereotaxis in China...
People with pacemakers or defibrillators who experience only short episodes of atrial fibrillation have a very low risk of stroke, suggesting that anticoagulants in this group of patients were not likely to reduce the risk for stroke, according to...
Coupling data mining of adverse event reports and electronic health records with targeted laboratory experiments, researchers have found a way to identify and confirm previously unknown drug interactions, according to a study published in the Journal of the American...
Analyses from the FIRE AND ICE trial has shown lower costs with cryoballoon catheter ablation compared to radiofrequency ablation—related to trial period—as a result of fewer cardiovascular rehospitalisations and repeat ablations.
The data were presented at a late-breaking clinical trial...
Medtronic has announced US Food and Drug Administration (FDA) approval for its suite of cardiac rhythm and heart failure devices and leads to be scanned in both 3 and 1.5 Tesla (T) magnetic resonance imaging (MRI) machines.
This advancement gives...
The Food and Drug Administration has recently issued a safety advisory communication regarding premature battery depletion associated with lithium deposits for some implantable cardioverter defibrillators (ICDs) and cardiac resynchronisation therapy defibrillators (CRT-D) from St Jude Medical.
The devices addressed in...
SentreHEART has announced it has treated the first patients using the Eclipse surgical device for left atrial appendage (LAA) closure. Krzysztof Bartus (Jagiellonian University, Department of Cardiovascular and Transplant Surgery in Krakow, Poland) performed the procedures.
“The procedures went exceedingly...
CVRx has appointed Tom Moore to the newly created, officer position of vice president, US Market Development. In this role, Moore will lead all field efforts related to the successful completion of the BeAT-HF (Baroreflex activation therapy for heart failure) pivotal clinical trial. Additionally, he...
Heart Rhythm Congress (HRC), the UK’s largest educational congress devoted solely to heart rhythm disorders will gather over 3,000 heart rhythm experts and healthcare professionals from around the world from 9‒12 October 2016 in Birmingham, UK. HRC aims to...
With the aim to help prevent atrial fibrillation (AF) and to improve its management worldwide— particularly in low- and middle-income countries—the World Heart Federation (WHF) has launched a new Roadmap to tackle non-valvular AF.
The Roadmap or global implementation strategy...
Commonly used prescribed painkillers for treating pain and inflammation are associated with an increased risk of hospital admission for heart failure, finds a study recently published in The BMJ.
The drugs include traditional non-steroidal anti-inflammatory drugs (NSAIDs) as well as...
Mitac Europe has announced the launch of its MiCor A100, an electrocardiograph wristband that measures heart rhythm and highlights symptoms of cardiac conduction abnormalities.
According to a company release, the MiCor A100 is an easy to use and can measure...
CardioFocus has announced the first commercial US procedure using its HeartLight Endoscopic Ablation system for the treatment of patients with paroxysmal atrial fibrillation (AF) at The Mount Sinai Hospital in New York City. The HeartLight System received premarket approval...
The European Heart Academy of the European Society of Cardiology (ESC), the European Heart Rhythm Association (EHRA) and the Maastricht University Medical Centre are launching the European Diploma of Advanced Studies in Cardiac Arrhythmia Management (DAS-CAM). A combined component...
Scientists from Johns Hopkins University and University of Bonn in Germany have found that beams of light could potentially replace electric shocks to terminate ventricular arrhythmias.
A new risk score, based on data from GARFIELD-AF (Global anticoagulation registry in the field), may be superior to the CHA2DS2-VASc score at predicting which patients truly have a low-risk of stroke. Therefore, the score—a simplified form of which...
The Journal of the American College of Cardiology (JACC) has retracted the paper titled: “Impact of rotor ablation in non-paroxysmal atrial fibrillation patients: Results from the randomized OASIS trial”, which was published in July.
According to an announcement published on...
Two randomised clinical trials presented at a Hot Line session of the European Society of Cardiology Congress (ESC; 27‒31 August, Rome, Italy) have shown that remote monitoring of cardiac implantable electronic devices (CIEDs) did not reduce mortality or hospitalisations...
Despite the common perception that moderate alcohol intake is good for the heart, new research suggests long-term alcohol consumption, even as little as one drink a day may enlarge the heart’s atrium and increase the risk of developing atrial fibrillation,...
EBR Systems has announced the US Food and Drug Administration (FDA) has granted an Investigational Device Exemption (IDE) for its WiSE (Wireless Stimulation Endocardially) technology for cardiac resynchronization therapy (CRT). This IDE enables EBR Systems to start a major US study—SOLVE CRT—to...
Cryoballoon ablation for patients with diagnosed atrial fibrillation, and long-term cardiac monitoring for survivors of stroke who do not have an established diagnosis of atrial fibrillation have both been recognised in updated guidelines published by the European Society of Cardiology...
Given the early in-human experience with leadless pacemakers, safety and effectiveness of chronic retrieval of such devices is still under question. Reinoud Knops (Amsterdam, The Netherlands) reviews what is known so far on this subject.
By Reinoud Knops
Since...
Results from the first study to compare outcomes of two left atrial appendage closure devices—Watchman (Boston Scientific) and Amplatzer (St Jude Medical)—indicate that there are no significant differences between the devices. Authors Filippo Figini (Interventional Cardiology Department, San Raffaele...
The St Jude Medical Amplatzer Amulet Investigational Device Exemption (IDE) trial, which will evaluate the safety and effectiveness of the company’s Amplatzer Amulet left atrial appendage occluder used to close the left atrial appendage (LAA) in patients diagnosed with...
Risk of death from a sudden loss of heart function has been found to be significantly greater in patients free thyroxine (FT4) levels at the higher end of normal range, compared to patients with levels at the lower end,...
ENSURE-AF, the largest prospective, randomised clinical trial to date of anticoagulation for electrical cardioversion in non-valvular atrial fibrillation, has shown that edoxaban is an effective and safe alternative to vitamin K antagonists (VKAs) for the prevention of stroke and...
Implantable cardioverter-defibrillators (ICDs) do not improve overall long-term survival compared to medical treatment in patients with non-ischaemic systolic heart failure, according to results of the DANISH clinical trial. These data prompted researchers and other physicians at the European Society...
Atrial fibrillation is associated with a wide range of serious events, including heart attacks, heart failure, chronic kidney disease, and sudden cardiac death, a large study in The British Medical Journal has found.
The findings show that the risk associated...
The first European Society of Cardiology (ESC) Guidelines on Atrial Fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) have been published in the European Heart Journal and the European Journal of Cardio-Thoracic Surgery, and on...
More than three years of nightly treatment with a continuous positive airway pressure (CPAP) machine did not reduce cardiovascular risk more than usual care among patients with cardiovascular disease and obstructive sleep apnoea.
Findings from the Sleep Apnea Cardiovascular Endpoints...
A coalition of charities, patient groups, professional bodies and healthcare companies with an interest in heart failure has been established to raise the profile of the condition within the UK government, the UK National Health Service and the media....
The European Heart Rhythm Association (EHRA) and the EP Europace journal have released the supplement to its ninth annual EHRA White Book, developed in partnership with Biotronik.
The 2016 EP Europace Supplement is intended to provide deeper analysis into data...
The Journal of the American College of Cardiology: Clinical Electrophysiology has published an article investigating BioSig Technologies’ Pure EP system.
According to a company release, the publication highlights Pure EP's broader dynamic range and sampling rate versus standard EP recording...
EPIC Alliance experts at the 2016 European Society of Cardiology (ESC; Rome, Italy) congress presented original research and real-world clinical evidence on non-responders to cardiac resynchronisation therapy and atrial fibrillation ablation today.
The all-women panel of physicians from the EPIC...
During a scientific talk at the 2016 European Society of Cardiology (ESC) Congress, Gerhard Hindricks, Leipzig Heart Center, Germany, presented results of a meta-study; the TRUECOIN study, pooling 2,405 patients from the TRUST, ECOST and IN-TIME trials. Biotronik Home...
Daiichi Sankyo has announced data from new sub-analyses of the Prevention of thromboembolic events—European Registry in Atrial Fibrillation (PREFER in AF) patient registry, which reveal at-risk patient groups, current treatment gaps in the management of atrial fibrillation and trends...
The Cardiac Resynchronization Therapy Efficacy Enhancement (CRTee) study has shown that the Medtronic EffectivCRT-during atrial fibrillation algorithm can improve therapy delivery in heart failure patients with atrial fibrillation. This first-of-its-kind data on the algorithm were presented at the 2016...
Portola Pharmaceuticals has announced interim results from the ongoing phase3b/4 ANNEXA-4 study of andexanet alfa (AndexXa), a Factor Xa inhibitor antidote.
In this study of patients with Factor Xa inhibitor-associated acute major bleeding, a preliminary analysis of interim data from...
The number of cardiovascular drugs in the research pipeline has declined across all phases of development in the last 20 years—even as cardiovascular disease has become the number one cause of death world-wide, according to research published in JACC:...
New long-term results from the Medtronic Micra Transcatheter System Global Clinical Trial have shown consistently low rates of major complication. The data—from largest and longest clinical evaluation of leadless pacing patients to date—was presented at a late-breaking trial at...
A growing body of evidence, including the most recent randomised clinical trial results presented at HRS and CARDIOSTIM-EHRA EUROPACE, has shown that cardiac resynchronisation therapy (CRT) with MultiPoint™ pacing technology from St. Jude Medical shows promise in improving response rates in...
Daiichi Sankyo has announced it will present the first results from ENSURE-AF, the largest prospective randomised clinical trial to date evaluating a non-vitamin K antagonist oral anticoagulant (edoxaban) compared to a current standard of care in patients with non-valvular...
Researchers in the USA have identified the first generalisable risk score for sudden cardiac death. This prediction model, the researchers argue, provides well-calibrated, absolute risk estimates across different risk strata in an adult population without a history of cardiovascular...
Cambridge Design Partnership recently announced it is working with Kings College London to further develop a new steerable catheter designed to treat complex cardiac arrhythmias such as atrial fibrillation and ventricular tachycardia. The catheter, created by a group of...
Bristol-Myers Squibb and Pfizer have announced that 19 abstracts (late-breaking, rapid-fire, oral and poster presentations) on apixaban (Eliquis) will be presented at the European Society of Cardiology Congress (ESC; 27–31 August, Rome, Italy).
These new data include post-hoc analyses from...
New analyses from the Global Anticoagulant Registry in the Field-Atrial Fibrillation (GARFIELD-AF) will be presented at the European Society of Cardiology (ESC) Congress (27‒31 August, Rome, Italy) with a focus on the importance of risk stratification and tailored management...
This educational supplement is only for readers in countries outside France, Japan and the USA
In this educational supplement, sponsored by Boston Scientific, Cardiac Rhythm News explores left atrial appendage closure with the WATCHMANTM device. Contents include:
Is oral anticoagulation the right treatment for stroke...
Researchers in the United Kingdom (working with colleagues in Connecticut, France and India) examined 186,151 non-adopted participants aged 55‒73 years with deceased parents who participated in UK Biobank, a project that collects data on volunteers for health research. Follow-up...
ARCA Biopharma has announced that the 100th patient has been randomised into GENETIC-AF, a seamless design phase 2B/3 clinical trial evaluating Gencaro (bucindolol hydrochloride) as a potential treatment for atrial fibrillation. This represents two-thirds of the minimum number of...
Boehringer Ingelheim has announced the launch of its global RE-VECTO programme for idarucizumab (Praxbind), the specific reversal agent for dabigatran etexilate (Pradaxa).
Idarucizumab is approved to reverse the anticoagulant effects of dabigatran in rare critical care situations such as prior...
There is just a one in five chance that a potentially life-saving automated external defibrillator (AED) will be nearby when someone experiences cardiac arrest and a 20 to 30% chance that the nearby device will be inaccessible because it...
Biosense Webster has announced the US launch of the ThermoCool SmartTouch SF catheter. According to a company release, the catheter pairs contact force technology with a porous tip designed to optimise efficiency by providing uniform cooling at half the...
Boston Scientific has received US Food and Drug Administration (FDA) approval for the Emblem MRI subcutaneous implantable defibrillator (S-ICD) system, as well as magnetic resonance (MR) conditional labelling for all previously implanted EMBLEM S-ICD systems.
The new Emblem MRI...
According to a company release, the new device is ideal for maximum voltage guided ablation of atrial flutter.
The Cerablate Flutter is a newly designed radiofrequency-powered ablation catheter with an 8mm gold tip for the treatment of atrial flutter. The...
Following recent CE mark approval for Biotronik’s E-Series pacemakers, a UK patient has become the first in Europe to be implanted with the streamlined device.
The patient, a woman with a history of heart failure and complete heart block, had...
The ACTION Registry-GWTG in-hospital mortality risk model for heart attack patients has been updated to include cardiac arrest, and validated as a robust instrument for risk adjustment and benchmarking of mortality outcomes, according to a study published in the...
Recent results of the first randomised trial—OASIS trial—to compare rotor-only ablation with two other ablation strategies in non-paroxysmal atrial fibrillation patients found poor outcomes in terms of arrhythmia recurrence with rotor-only ablation. However, at CARDIOSTIM-EHRA EUROPACE (8‒11 June, Nice,...
A practice advisory update from the American Academy of Neurology (AAN) suggests that percutaneous patent foramen ovale (PFO) closure to patients with cryptogenic ischaemic stroke “should not be routinely offered outside of a research setting”.
However, in cases of patients...
Itamar Medical has received US Food and Drug Administration (FDA) clearance to expand the medical indication of WatchPAT for sleep apnoea diagnosis. WatchPAT is a home sleep test diagnostic device for sleep apnoea.
Under this approval, the use of WatchPAT...
Results from the RESPOND-CRT trial have shown a 35% risk reduction in hospitalisation at two years for heart failure patients implanted with cardiac resynchronisation therapy (CRT) devices equipped with the SonR haemodynamic sensor-guided feature (LivaNova).
Data were presented by Jagmeet...
Hanna E Bloomfield (Minneapolis VA Medical Center, Minneapolis, USA) and others report in Annals of Internal Medicine that limited evidence, from randomised control trials, indicates that a Mediterranean diet without a restriction on fat intake is associated with a reduced...
The US Food and Drug Administration (FDA) has released a draft guidance document entitled “Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices”.
According to an email from the consumer watchdog, the document is intended to clarify “how...
Stereotaxis has announced the market release of its second generation V-CAS Deflect catheter advancement system in Europe. The V-CAS Deflect catheter advancement system is designed to remotely control a proprietary robotic deflectable sheath during a cardiac ablation procedure with...
An analysis from the ENGAGE AF-TIMI 48 trial has found that sudden cardiac death was the single most common cause of death and accounted for about a third of all deaths and nearly half of all cardiovascular deaths amongst...
Cardiac imaging has emerged as an integral part of mapping and ablation in a variety of complex arrhythmias. Advances in imaging techniques, especially the multimodalities technique, have made it possible to map and ablate the more difficult and complex...
The current need for new preventative therapies of atrial fibrillation in chronic heart failure patients—with fewer side effects than currently available treatment strategies—has led ARCA biopharma to develop bucindolol hydrochloride (Gencaro), a pharmacologically unique beta-blocker and mild vasodilator. Cardiac...
Highlights:
-Ablation should be "preferred approach" in drug-resistant ventricular tachycardia
-First randomised trial shows "poor outcomes" with rotor ablation
-Guilherme Fenelon: Ablation in Chagas disease
-Pier Lambiase: EP data
-Advertorial: St. Jude Medical brings to market an automated, flexible and precise solution for cardiac mapping
-Profile:...
Highlights:
-Ablation should be "preferred approach" in drug-resistant ventricular tachycardia
-First randomised trial shows "poor outcomes" with rotor ablation
-Guilherme Fenelon: Ablation in Chagas disease
-Pier Lambiase: EP data
-Profile: Etienne Aliot
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/07/CRN-33_USA_low-res.pdf
Commonly used medications and nutritional supplements may cause or worsen heart failure, according to the first scientific statement from the American Heart Association to provide guidance on avoiding drug-drug or drug-condition interactions for people with heart failure.
The statement provides...
Stereotaxis has announced that Texas Children’s Hospital in Houston, USA, has installed the its Niobe ES system, which according to the company, is the only paediatric hospital in the country to offer the latest generation remote magnetic navigation platform for...
Royal Philips has launched the DreamMapper application and website in the UK, which is designed to assist people living with sleep apnoea to improve treatment adherence by providing tools and real time tracking and analysis of their positive airway...
Biotronik has received CE mark approval for its new Edora pacemakers and cardiac resynchronisation therapy pacemakers (CRT-Ps).Edora devices feature the Biotronik’s ProMRI (magnetic resonance imaging) technology, including the MRI AutoDetect sensor. According to a company release, the Edora pacemakers...
Patients with frequent premature ventricular complex and primary prevention implantable cardioverter defibrillator (ICD) indication who undergo ablation may be able to avoid ICD implant, according to research presented at CARDIOSTIM-EHRA EUROPACE (8‒11 June, Nice, France).
The paper, which has been...
A cohort study has found that medical devices approved first in the European Union (EU) are associated with a greater rate of post-marketing safety alerts and recalls compared with devices approved first in the USA.
First author Thomas J Hwang...
CorMatrix Cardiovascular has received CE mark approval for its CorMatrix CanGaroo ECM envelope. It is intended for use with cardiac implantable electronic devices including pacemakers and implantable cardioverter defibrillators. The company received US Food and Drug Administration clearance to...
MicroPort EP has recently received approval from the China Food and Drug Administration (CFDA) for Columbus 3D EP navigation system and FireMagic 3D irrigated ablation catheter. The approval of these products marks their official entrance to the Chinese market.
Columbus,...
The American College of Cardiology (ACC) and the American Heart Association (AHA) have released updated clinical performance and quality measures for treating adult patients with atrial fibrillation or atrial flutter.
This document updates the previous measure set that was released in 2008...
Medtronic has entered into a definitive agreement to acquire HeartWare for approximately US$1.1 billion.
HeartWare’s flagship product, the HVAD system, features the world’s smallest full-support ventricular assist device (VAD) and is designed to reduce surgical invasiveness, improve patient recovery times...
The Texas Cardiac Arrhythmia Institute (TCAI) at St David’s Medical Center (Austin, USA) recently became the first facility in Texas to use the Micra transcatheter pacing system (TPS, Medtronic) after receiving US Food and Drug Administration approval.
The procedure was...
Catheter Robotics has completed the acquisition of a new product line to be named VIVO. The line is a computerised three-dimensional cardiac-mapping system showing electrical activation of the ventricles of the heart. VIVO stands for “view into ventricular onset”.
In...
Mobile devices, social media, visual media and crowdsourcing have the potential to improve emergency care for cardiac arrests, heart attacks and strokes, according to a new scientific statement from the American Heart Association.
The new statement, published in Circulation, reviewed...
Abbott has announced positive results from three clinical studies investigating the benefits associated with focal impulse rotor modulation (FIRM)-guided rotor ablation for atrial fibrillation.
The investigator-sponsored studies, which evaluated the use of Abbott’s RhythmView technology, were presented at CARDIOSTIM-EHRA EUROPACE...
The internet age has enabled patients to access a plethora of medical information. However, a potential drawback is that some of this information is inaccurate or misleading—causing unnecessary anxiety or false hope to patients. Adam Hartley reviews the advantages...
The Cardiac & Vascular Institute is the first organisation in the North Central Florida USA area of the USA to offer patients with non-valvular atrial fibrillation an alternative to long-term warfarin medication with the newly approved Watchman left atrial...
The first patient has been enrolled in the FROST study at William Beaumont Hospital, Dearborn, USA. This AtriCure-sponsored trial will evaluate intraoperative cryoanalgesia therapy using cryoablation in conjunction with standard of care (SOC) pain management compared to SOC alone.
The...
This advertorial is only available in countries outside the USA.
The EnSite Precision™ cardiac mapping system from St. Jude Medical is designed to provide automation, flexibility and precision for tailored treatment in patients with cardiac arrhythmias. This is a next-generation...
St Jude Medical has received an Innovation Award for its HeartMate 3 left ventricular assist system (LVAS) at the 2016 CARDIOSTIM EHRA EUROPACE International Congress of electrophysiology and cardiac technology.
Mark Carlson, chief medical officer at St Jude Medical and...
Late-breaking science sessions will be held for the first time at Frontiers in CardioVascular Biology (FCVB) 2016.
The meeting is organised by the Council on Basic Cardiovascular Science of the European Society of Cardiology (ESC) in collaboration with 13 European...
More than one-in-three patients with atrial fibrillation with an intermediate-to-high-risk of stroke are prescribed aspirin instead of oral anticoagulants, despite guidelines recommending the use of oral anticoagulants for this group of patients, according to a study published in the...
The world’s only wireless endocardial pacing system for cardiac resynchronisation therapy (CRT)—WiSE (Wireless Stimulation of the Endocardium) CRT System (EBR Systems)—is showing promise treating heart failure patients who have not previously responded to transvenous CRT and in those who...
Biotronik has launched its Solia S ProMRI, with a 5.6 French lead body. According to a company release, this is the smallest MR-conditional pacing lead available in the USA.
Solia S ProMRI is available in 45, 53 and 60 centimetre...
Over 260,000 people with atrial fibrillation (AF) in the UK are receiving either no preventative treatment, or are still taking only aspirin, no longer recommended by NICE (the National Institute for Health and Care Excellence) for the prevention of...
A study, which was presented at EuroPRevent 2016 (14–15 June, Sophia Antipolis, France), indicates that 15 minutes of daily exercise may be reasonable target for older adults. It found that such an exercise regimen was associated with a 22%...
New data from the FIRE AND ICE clinical trial have shown significantly fewer repeat ablations and lower hospitalisation rates for patients with paroxysmal atrial fibrillation treated with cryoballoon ablation compared to radiofrequency ablation.
The results were presented by Karl-Heinz Kuck...
The WEARIT-II prospective registry of more than 2,000 US patients has found over 90% survival rate at one-year in patients prescribed with the LifeVest wearable defibrillator (Zoll). Patients who experienced ventricular tachycardia/ventricular fibrillation (VT/VF) during use of the wearable...
Biotronik has won the Cardiostim Innovation Award in the category “Best Practice Improvement” for its MRI AutoDetect feature. The company’s Ilivia implantable cardioverter defibrillators (ICDs) and cardiac resynchronization device (CRT-Ds) are the world’s first equipped with a sensor capable of...
Results from several feasibility studies evaluating a new approach to implantable cardioverter defibrillator (ICD) therapy have been presented at CARDIOSTIM / EHRA EUROPACE (8-11 June; Nice, France) Medtronic has announced.
The Medtronic EV-ICD system, which currently is in development and...
New results from the Medtronic Micra Transcatheter Pacing System (TPS) global clinical trial have been presented in a late-breaking trial session at CARDIOSTIM / EHRA EUROPACE (8-11 June; Nice, France).
The follow-up data presented by Gabor Duray (head of Clinical...
We now live in an unprecedented era of technological advances, which is eclipsing our ability to test new therapies at the pace of these advances and the natural history of most heart rhythm disorders themselves. Many clinical trials have...
Karl-Heinz Kuck (Department of Cardiology, Asklepios Klinik St. Georg, Hamburg, Germany) has withdrawn his candidacy for president-elect of the European Society of Cardiology (ESC) and also resigned from board of the European Heart Rhythm Association (EHRA). This follows, as reported...
Highlights:
-No significant difference between “fire” and “ice” AF ablation
-INOVATE-HF shows no evidence that vagus nerve stimulation reduces death in heart failure
-James A Reiffel: Antiarrhythmic drugs
-WiSE CRT System: First implantations
- Profile: George Van Hare
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/32-Cardiac-Rhythm-News_low-res-.pdf
Highlights:
-Ultrasound-based system shows promise mapping atrial fibrillation
- New survey on cardiac resynchronisation therapy use in Europe launched
- Focus on: Leadless pacing
- EHRA White Book
- Allessandro Capucci: Energy drinks
- Profile: Jonathan M Kalman
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/31-Cardiac-Rhythm-News_low-res.pdf
Highlights:
-Benznidazole ineffective at reducing clinical progression in chronic Chagas disease
- Idarucizumab immediately reverses anticoagulant effect of dabigatran in AF patients
- EHRA inventors awards
- Enrico Caiani: Safety hearlth apps
- Profile: Michael Glikson
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/30-Cardiac-Rhythm-News_low-res.pdf
Highlights:
-Botox reduces AF burden for up to a year after cardiac surgery
-Safety and efficacy of Transcatheter Pacing System demonstrated in initial study
-Maria G Bongiorni: Lead extraction
-Elad Anter: Sleep apnoea
-Profile: Cecilia Linde
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/29-Cardiac-Rhythm-News_low-res.pdf
Highlights:
-Reversal agents may help to quell fears of major bleeding with novel oral anticoagulants
-Baroreflex neuromodulation therapy shows positive outcomes in heart failure treatment
-Laurent Macle: PV isolation
-Heart failure: New therapies
-Profile: Michael Gold
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/28-Cardiac-Rhythm-News_low-res_EU.pdf
Highlights:
-Reversal agents may help to quell fears of major bleeding with novel oral anticoagulants
-Baroreflex neuromodulation therapy shows positive outcomes in heart failure treatment
-Laurent Macle: PV isolation
-Heart failure: New therapies
-Profile: Michael Gold
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/28-Cardiac-Rhythm-News_low-res_USA.pdf
Highlights:
-Patients with standard cardiac devices can undergo non-thoracic MRI scans
-Antiplatelets combined with anticoagulants linked to increased risk of dementia
-Michel Haïssaguerre: Non-invasive mapping
-Adrian Zurbucken: Non-battery Pacemaker
-Profile: Hung-Fat Tse
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/27-Cardiac-Rhythm-News_low-res_EU.pdf
Highlights:
-Patients with standard cardiac devices can undergo non-thoracic MRI scans
-Antiplatelets combined with anticoagulants linked to increased risk of dementia
-Michel Haïssaguerre: Non-invasive mapping
-Adrian Zurbucken: Non-battery Pacemaker
-Profile: Hung-Fat Tse
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/27-Cardiac-Rhythm-News_low-res_USA.pdf
Highlights:
-First sham controlled trial of vagal nerve stimulation for heart failure fails
-New drug shows promise for treating heart failure
-Boris Schmidt: Left atrial appendage
-Felipe Bisbal: Cardiac imaging
-Profile: Massimo Santini
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/26-Cardiac-Rhythm-News_low-res.pdf
Highlights:
-First sham controlled trial of vagal nerve stimulation for heart failure fails
-New drug shows promise for treating heart failure
-Boris Schmidt: Left atrial appendage
-Felipe Bisbal: Cardiac imaging
-Profile: Massimo Santini
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/26-Cardiac-Rhythm-News_USA_low-res.pdf
Highlights:
-Defibrillation testing may not be necessary
-Aggressive lifestyle management helps improve success rate of AF after ablation
-John D Day: AF management
-Feature: leadless pacing
-Profile: Nick Linker
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/25-Cardiac-Rhythm-News.pdf
Highlights:
-Early CRT reduces risk of death in selected mild heart failure patients
-Cardiac monitors are better than standard monitoring detecting AF in cryptogenic stroke patients
-HeartLight for AF: Featured product
-Eric Prystowsky: AF therapies
-Profile: Richard Fogel
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/24-Cardiac-Rhythm-News.pdf
Highlights:
-Largest anticoagulant trial in atrial fibrillation shows edoxaban is as effective as warfarin
-New generation pacemakers reduce atrial fibrillation progression by 61% at two years
-Ablation of persistent atrial fibrillation, are we there yet?
-Profile: Anne Curtis
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/23-Cardiac-Rhythm-News.pdf
Highlights:
-Resynchronisation in narrow QRS patients questioned
-Delayed enhancement
-MRI detected atrial fibrosis could predict successful ablation in atrial fibrillation
-Clinical strategies for selecting oral anticoagulants in atrial fibrillation patients
-Profile: Antonio Raviele
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/22-Cardiac-Rhythm-News-.pdf
Highlights:
-Wearable defibrillator aids decision making on ICD need
-First-in-man study shows promise with leadless pacing
-Remote monitoring of ICD patients: What is the patients' opinion by Werner Jung
-Profile: Andreas Goette
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/21-Cardiac-Rhythm-News.pdf
Highlights:
- Renal denervation now under consideration for arrhythmias
- Watchman device is safe but efficacy debated
- Leadless technology: The next frontier of cardiac pacing
- Profile: John D Day
https://cardiacrhythmnews.com/wp-content/uploads/sites/12/2016/06/20-Cardiac-Rhythm_low-res.pdf
Etienne Aliot (Nancy, France) has always been fascinated by the work of surgeons. This fascination set him on his path through medical school. He has since been at the forefront of many electrophysiological research initiatives, including influential consensus documents...
Stereotaxis is to present Niobe ES magnetic navigation system and supporting clinical evidence at Cardiostim-EHRA Europace 2016, taking place June 8-11 in Nice, France.
William C Mills, Stereotaxis chief executive officer, says, “This year, we have enhanced the ‘Stereotaxis experience’...
St Jude Medical’s CardioMEMS heart failure (HF) system has been added to the European Society of Cardiology (ESC) guidelines as a directed therapy management and monitoring tool for heart failure patients. The new 2016 ESC Clinical Practice Guidelines for...
The European Heart Rhythm Association (EHRA) is launching the patient- and caregiver-oriented website afibmatters.org in Swedish and Portuguese.
Afibmatters.org aims to contribute to improvements in the quality of life and health of patients with atrial fibrillation. The website is currently...
The first two patients have been treated with CVRx’s Barostim Neo system in the Baroreflex Activation Therapy for Heart Failure pivotal clinical trial (BEAT-HF). The procedures were done at the University of California San Francisco (San Francisco, USA) and...
The US Food and Drug Administration (FDA) has finalised its efforts to streamline the “compassionate use” process, used by physicians to access investigational drugs and biologics for patients with limited treatment options.
A statement from Robert Califf, the deputy...
Auris Surgical Robotics and Hansen Medical have signed a definitive merger agreement under which Auris will acquire Hansen Medical for US$4.00 per share in cash, or a total equity value of approximately US$80 million.
The transaction is expected to close...
A new bill introduced to the US Senate by Senator Dan Coats seeks to address the gap between the US Food and Drug Administration (FDA)’s priority review process for breakthrough medical devices, and the Centers for Medical and Medicare...
Occlutech has obtained European CE mark approval for its left atrial appendage, (LAA), occluder.
The device is a specifically designed implant for the minimally invasive closure of the LAA, a procedure that minimises the risk of strokes in patients...
Stereotaxis has announced the results of a retrospective study conducted at Paracelsus Medical University in Nuremberg, Germany, which showed significantly reduced radiation exposure for patients undergoing catheter ablation for persistent atrial fibrillation with the Niobe magnetic navigation system. The...
Pre-clinical data, presented at the 13th International Dead Sea Symposium on Innovations in Cardiac Arrhythmias and Device Therapy (IDSS; 6–9 March, Tel Aviv, Israel), have shown that it is feasible and safe to isolate pulmonary veins for the treatment...
There is a lack of a uniform approach or guidelines when dealing with the ethical, legal and privacy concerns that can arise in cardiogenetics. These concerns include “duty to warn” and “child protection” dilemmas and may occur in both...
New research indicates that catheter ablation may achieve greater long-term freedom from atrial fibrillation, as well as a reduction in unplanned hospitalisation and mortality in patients with heart failure and persistent atrial fibrillation.
In this open-label, randomised, parallel-group, multicentre clinical...
The Netherlands presidency of the European Council and representatives of the European Parliament have reached a political agreement on two draft regulations for medical devices. The new regulations are aimed at ensuring that medical devices and in vitro diagnostic...
The heart rate may be an indicator of a person’s life expectancy. A research team at the Technical University of Munich (TUM) (Munich, Germany) has to this end analysed an effect which at first seems paradoxical: Minor irregularities in...
Maria Lucia Narducci (Department of Cardiovascular Sciences, Catholic University of Sacred Heart, Rome, Italy) and others report in Europace that the presence of residual fibrous tissue—or “ghosts”—after transvenous lead extraction is associated with a significantly increased risk of death....
Syed Rafay A Sabzwari and Dhanunjaya Lakkireddy (Kansas City, USA) review the importance of obstructive sleep apnoea (OSA) in electrophysiology and cardiology at large, as a secondary impact to an underserved area of healthcare, which also provides practical implications...
Biotronik has launched CardioMessenger Smart in the USA. CardioMessenger Smart is a portable monitoring device, about the size of a smartphone, which makes data from pacemaker, implantable cardioverter defibrillator (ICD), and insertable cardiac monitor (ICM) patients available remotely to...
A prospective, multicentre study—THERMOCOOL VT—analysing a cohort of non-randomised patients, published in February in the Journal of the American College of Cardiology, has shown that radiofrequency catheter ablation dramatically reduces ventricular tachycardia episodes and improves quality of life at...
Catheter Robotics has completed the acquisition of a new product line to be named VIVO (View Into Ventricular Onset). VIVO is a computerised three-dimensional cardiac mapping system designed to show electrical activation of the ventricles of the heart.Catheter Robotics...
ResMed has announced primary results from a multicentre, randomised controlled phase II trial—CAT-HF—presented at the European Society of Cardiology’s 2016 Annual Heart Failure Congress.
CAT-HF assessed whether the treatment of moderate to severe sleep-disordered breathing (obstructive or central sleep apnoea)...
A randomised controlled study has found that yoga with light movements and deep breathing may lead to improved quality of life, as well as reduced blood pressure and heart rate in patients with paroxysmal atrial fibrillation. The study findings...
A subsequent study of the Value PVI (pulmonary vein isolation) study report several economic benefits, including reduced staff overtime and more time remaining for additional usage of electrophysiology laboratory resources with the use of cryoballoon ablation for atrial fibrillation...
New data from the EWOLUTION registry, presented at EuroPCR 2016, confirms safety of the Boston Scientific left atrial appendage closure system (Watchman). The data are for more than 1,000 patients, from across Europe, who received the device and focus on...
St Jude Medical has announced results from two cardiovascular clinical trials presented at EuroPCR 2016. The studies, which look at how St Jude Medical’s fractional flow reserve (FFR) technology impacts patient outcomes in acute coronary syndrome and a comparison...
Karl-Heinz Kuck, Asklepios Klinik St Georg, Hamburg, Germany, speaks at ACC 2016 about the FIRE AND ICE trial results which have shown that cryoballoon ablation is non-inferior to radiofrequency ablation in treating patients with drug-refractory paroxysmal atrial fibrillation, with...
Guilherme Fenelon (Sao Paulo, Brazil) overviews the role of ablation of ventricular tachycardia in Chagas disease. He says: catheter ablation in Chagas disease remains a “challenging procedure,” however, substrate mapping and ablation of ventricular tachycardia “is very useful for...
Corvia Medical has been granted CE mark approval for its InterAtrial shunt device (IASD). The IASD is a transcatheter device designed to treat heart failure with preserved ejection fraction (HFpEF), previously called diastolic heart failure.
David Muller, director of Cardiac...
Implantable cardioverter-defibrillators (ICDs) do not improve overall long-term survival compared to medical treatment in patients with non-ischaemic systolic heart failure, according to results of the DANISH clinical trial. These data prompted researchers and other physicians at the European Society...
OASIS, the first randomised, controlled, multicentre clinical trial to compare rotor-only ablation with two other ablation strategies in non-paroxysmal atrial fibrillation patients found “poor outcomes in terms of arrhythmia recurrence” with rotor-only ablation, for which enrolment on this treatment...